(tofacitinib)
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
14.1 Clinical Studies in Rheumatoid Arthritis
The rheumatoid arthritis (RA) clinical development program with XELJANZ tablets (referred to as “XELJANZ” in this subsection of labeling) included six randomized controlled trials in adults with moderate to severe active RA.
Trial Design
- •
- Study RA-I (NCT00814307) was a 6-month monotherapy trial in which 610 patients with moderate to severe active RA who had an inadequate response to a DMARD (nonbiologic or biologic) received XELJANZ 5 mg or 10 mg twice daily or placebo added to their background DMARD. At the Month 3 visit, all patients randomized to placebo treatment were switched in a blinded fashion to a second predetermined treatment of XELJANZ 5 mg or 10 mg twice daily. The primary endpoints at Month 3 were the proportion of patients who achieved an ACR20 response, changes in Health Assessment Questionnaire – Disability Index (HAQ-DI), and rates of Disease Activity Score DAS28-4(ESR) less than 2.6.
- •
- Study RA-II (NCT00856544) was a 12-month trial in which 792 patients with moderate to severe active RA who had an inadequate response to a nonbiologic DMARD received XELJANZ 5 mg or 10 mg twice daily or placebo added to background DMARD treatment (excluding potent immunosuppressive treatments such as azathioprine or cyclosporine). At the Month 3 visit, nonresponding patients were switched in a blinded fashion to a second predetermined treatment of XELJANZ 5 mg or 10 mg twice daily. At the end of Month 6, all patients treated with placebo were switched to their second predetermined XELJANZ treatment in a blinded fashion. The primary endpoints were the proportion of patients who achieved an ACR20 response at Month 6, changes in HAQ-DI at Month 3, and rates of DAS28-4(ESR) less than 2.6 at Month 6.
- •
- Study RA-III (NCT00853385) was a 12-month trial in 717 patients with moderate to severe active RA who had an inadequate response to methotrexate (MTX). Patients received XELJANZ 5 mg or 10 mg orally twice daily, adalimumab 40 mg subcutaneously every other week, or placebo added to background MTX. Patients treated with placebo were switched as in Study RA-II. The primary endpoints were the proportion of patients who achieved an ACR20 response at Month 6, HAQ-DI at Month 3, and DAS28-4(ESR) less than 2.6 at Month 6.
- •
- Study RA-IV (NCT00847613) was a 2-year trial with a planned analysis at 1 year in which 797 patients with moderate to severe active RA who had an inadequate response to MTX received XELJANZ 5 mg or 10 mg twice daily or placebo added to background MTX. Patients treated with placebo were switched as in Study RA-II. The primary endpoints were the proportion of patients who achieved an ACR20 response at Month 6, mean change from baseline in van der Heijde-modified total Sharp Score (mTSS) at Month 6, HAQ-DI at Month 3, and DAS28-4(ESR) less than 2.6 at Month 6.
- •
- Study RA-V (NCT00960440) was a 6-month trial in which 399 patients with moderate to severe active RA who had an inadequate response to at least one approved TNF blocking biological product received XELJANZ 5 mg or 10 mg twice daily or placebo added to background MTX. At the Month 3 visit, all patients randomized to placebo treatment were switched in a blinded fashion to a second predetermined treatment of XELJANZ 5 or 10 mg twice daily. The primary endpoints at Month 3 were the proportion of patients who achieved an ACR20 response, HAQ-DI, and DAS28-4(ESR) less than 2.6.
- •
- Study RA-VI (NCT01039688) was a 2-year monotherapy trial with a planned analysis at 1 year in which 952 MTX-naïve patients with moderate to severe active RA received XELJANZ 5 or 10 mg twice daily or MTX dose-titrated over 8 weeks to 20 mg weekly. The primary endpoints were mean change from baseline in van der Heijde-modified Total Sharp Score (mTSS) at Month 6 and the proportion of patients who achieved an ACR70 response at Month 6.
Although other dosages have been studied, the recommended dosage of XELJANZ is 5 mg twice daily. XELJANZ 10 mg twice daily is not recommended for the treatment of RA [see Dosage and Administration (2.3)].
Clinical Response
The percentages of XELJANZ-treated patients who achieved ACR20, ACR50, and ACR70 responses in Studies RA-I, IV, and V are shown in Table 10. Similar results were observed with Studies RA-II and III. In trials RA-I through V, patients treated with 5 mg twice daily XELJANZ had higher ACR20, ACR50, and ACR70 response rates versus patients treated with placebo, with or without background DMARD treatment, at Month 3 and Month 6. Higher ACR20 response rates were observed within 2 weeks compared to placebo. In the 12-month trials, ACR response rates in XELJANZ-treated patients were consistent at 6 and 12 months.
| ||||||
Monotherapy in Nonbiologic or Biologic DMARD Inadequate Responders* | MTX Inadequate Responders† | TNF Blocker Inadequate Responders‡ | ||||
Study RA-I | Study RA-IV | Study RA-V | ||||
N§ | Placebo + background DMARD | XELJANZ 5 mg Twice Daily + background DMARD | Placebo + background MTX | XELJANZ 5 mg Twice Daily + background MTX | Placebo + background MTX | XELJANZ 5 mg Twice Daily + background MTX |
122 | 243 | 160 | 321 | 132 | 133 | |
ACR20 Month 3 Month 6 | 26% NA¶ | 59% 69% | 27% 25% | 55% 50% | 24% NA | 41% 51% |
ACR50 Month 3 Month 6 | 12% NA | 31% 42% | 8% 9% | 29% 32% | 8% NA | 26% 37% |
ACR70 Month 3 Month 6 | 6% NA | 15% 22% | 3% 1% | 11% 14% | 2% NA | 14% 16% |
In Study RA-IV, a greater proportion of patients treated with XELJANZ 5 mg twice daily plus MTX achieved a low level of disease activity as measured by a DAS28-4(ESR) less than 2.6 at 6 months compared to those treated with MTX alone (Table 11).
Study RA-IV | ||
DAS28-4(ESR) Less Than 2.6 | Placebo + MTX 160 | XELJANZ 5 mg Twice Daily + MTX 321 |
Proportion of responders at Month 6 (n) | 1% (2) | 6% (19) |
Of responders, proportion with 0 active joints (n) | 50% (1) | 42% (8) |
Of responders, proportion with 1 active joint (n) | 0 | 5% (1) |
Of responders, proportion with 2 active joints (n) | 0 | 32% (6) |
Of responders, proportion with 3 or more active joints (n) | 50% (1) | 21% (4) |
The results of the components of the ACR response criteria for Study RA-IV are shown in Table 12. Similar results were observed for XELJANZ in Studies RA-I, II, III, V, and VI.
Study RA-IV | ||||
Placebo + MTX N=160 | XELJANZ 5 mg Twice Daily + MTX N=321 | |||
Component (mean)* | Baseline | Month 3* | Baseline | Month 3* |
Number of tender joints (0-68) | 23 (13) | 18 (14) | 24 (14) | 13 (14) |
Number of swollen joints (0-66) | 14 (9) | 10 (9) | 14 (8) | 6 (8) |
Pain† | 55 (24) | 47 (24) | 58 (23) | 34 (23) |
Patient global assessment† | 54 (23) | 47 (24) | 58 (24) | 35 (23) |
Disability index (HAQ-DI)‡ | 1.32 (0.67) | 1.19 (0.68) | 1.41 (0.68) | 0.99 (0.65) |
Physician global assessment† | 56 (18) | 43 (22) | 59 (16) | 30 (19) |
CRP (mg/L) | 13.7 (14.9) | 14.6 (18.7) | 15.3 (19.0) | 7.1 (19.1) |
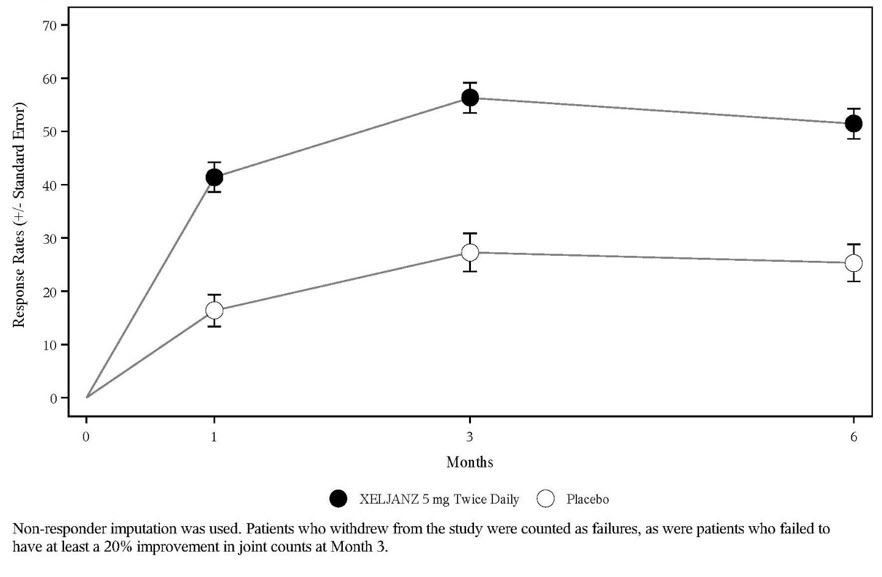
The percent of ACR20 responders by visit for Study RA-IV is shown in Figure 4. Similar responses were observed for XELJANZ in Studies RA-I, II, III, V, and VI.
Figure 4: Percentage of ACR20 Responders by Visit Through Month 6 in Study RA-IV
Radiographic Response
Two studies were conducted to evaluate the effect of XELJANZ on structural joint damage. In Study RA-IV and Study RA-VI, progression of structural joint damage was assessed radiographically and expressed as change from baseline in mTSS and its components, the erosion score and joint space narrowing score, at Months 6 and 12. The proportion of patients with no radiographic progression (mTSS change less than or equal to 0) was also assessed.
In Study RA-IV, XELJANZ 5 mg twice daily reduced the mean progression of structural damage (not statistically significant) as shown in Table 13. Analyses of erosion and joint space narrowing scores were consistent with the overall results.
In the placebo plus MTX group, 74% of patients experienced no radiographic progression at Month 6 compared to 84% of patients treated with XELJANZ plus MTX 5 mg twice daily.
In Study RA-VI, XELJANZ monotherapy inhibited the progression of structural damage compared to MTX at Months 6 and 12 as shown in Table 13. Analyses of erosion and joint space narrowing scores were consistent with the overall results.
In the MTX group, 55% of patients experienced no radiographic progression at Month 6 compared to 73% of patients treated with XELJANZ 5 mg twice daily.
Study RA-IV | |||
Placebo | XELJANZ 5 mg Twice Daily | XELJANZ 5 mg Twice Daily | |
mTSS‡ | |||
Baseline | 33 (42) | 31 (48) | - |
Month 6 | 0.5 (2.0) | 0.1 (1.7) | -0.3 (-0.7, 0.0) |
Study RA-VI | |||
MTX | XELJANZ 5 mg Twice Daily | XELJANZ 5 mg Twice Daily | |
mTSS‡ | |||
Baseline | 17 (29) | 20 (40) | - |
Month 6 | 0.8 (2.7) | 0.2 (2.3) | -0.7 (-1.0, -0.3) |
Month 12 | 1.3 (3.7) | 0.4 (3.0) | -0.9 (-1.4, -0.4) |
Physical Function Response
Improvement in physical functioning was measured by the HAQ-DI. Patients who received XELJANZ 5 mg twice daily demonstrated greater improvement from baseline in physical functioning compared to patients who received placebo at Month 3.
The mean (95% CI) difference from placebo in HAQ-DI improvement from baseline at Month 3 in Study RA-III was -0.22 (-0.35, -0.10) in patients who received 5 mg XELJANZ twice daily. Similar results were obtained in Studies RA-I, II, IV and V. In the 12-month trials, HAQ-DI results in XELJANZ-treated patients were consistent at 6 and 12 months.
Other Health-Related Outcomes
General health status was assessed by the Short Form health survey (SF-36). In Studies RA-I, IV, and V, patients who received XELJANZ 5 mg twice daily demonstrated greater improvement from baseline compared to placebo in physical component summary (PCS), mental component summary (MCS) scores and in all 8 domains of the SF-36 at Month 3.
14.2 Clinical Studies in Psoriatic Arthritis
The psoriatic arthritis (PsA) clinical development program with XELJANZ tablets (referred to as “XELJANZ” in this subsection of labeling) included 2 multicenter, randomized, double-blind, placebo-controlled trials in 816 adults with active PsA (Studies PsA-I and PsA-II).
Trial Designs and Population
All patients had active PsA for at least 6 months based upon the Classification Criteria for Psoriatic Arthritis (CASPAR), at least 3 tender/painful joints and at least 3 swollen joints, and active plaque psoriasis. Patients randomized and treated across the 2 clinical trials represented different PsA subtypes at screening, including <5 joints or asymmetric involvement (21%), ≥5 joints involved (90%), distal interphalangeal (DIP) joint involvement (61%), arthritis mutilans (8%), and spondylitis (19%). Patients in these clinical trials had a diagnosis of PsA for a mean (SD) of 7.7 (7.2) years. At baseline, 80% and 53% of patients had enthesitis and dactylitis, respectively. At baseline, all patients were required to receive treatment with a stable dose of a nonbiologic DMARD (79% received methotrexate, 13% received sulfasalazine, 7% received leflunomide, 1% received other nonbiologic DMARDs). In both clinical trials, the primary endpoints were the ACR20 response and the change from baseline in HAQ-DI at Month 3.
- •
- Study PsA-I was a 12-month clinical trial in 422 patients who had an inadequate response to a nonbiologic DMARD (67% and 33% were inadequate responders to 1 nonbiologic DMARD and ≥2 nonbiologic DMARDs, respectively) and who were naïve to treatment with a TNF blocker. Although Study PsA-1 included patients who are TNF blocker-naïve, XELJANZ and XELJANZ XR are not approved for use in TNF blocker-naïve patients [see Indications and Usage (1.2)]. Patients were randomized in a 2:2:2:1:1 ratio to receive XELJANZ 5 mg twice daily, XELJANZ 10 mg twice daily, adalimumab 40 mg subcutaneously once every 2 weeks, placebo to XELJANZ 5 mg twice daily treatment sequence, or placebo to XELJANZ 10 mg twice daily treatment sequence, respectively; study drug was added to background nonbiologic DMARD treatment. At the Month 3 visit, all patients randomized to placebo treatment were switched in a blinded fashion to a predetermined XELJANZ dosage of 5 mg or 10 mg twice daily. Study PsA-I was not designed to demonstrate noninferiority or superiority to adalimumab.
- •
- Study PsA-II was a 6-month clinical trial in 394 patients who had an inadequate response to at least 1 approved TNF blocker (66%, 19%, and 15% were inadequate responders to 1 TNF blocker, 2 TNF blockers and ≥3 TNF blockers, respectively). Patients were randomized in a 2:2:1:1 ratio to receive XELJANZ 5 mg twice daily, XELJANZ 10 mg twice daily, placebo to XELJANZ 5 mg twice daily treatment sequence, or placebo to XELJANZ 10 mg twice daily treatment sequence, respectively; study drug was added to background nonbiologic DMARD treatment. At the Month 3 visit, placebo patients were switched in a blinded fashion to a predetermined XELJANZ dosage of 5 mg or 10 mg twice daily as in Study PsA-I.
Although other dosages have been studied, the recommended dosage of XELJANZ is 5 mg twice daily. XELJANZ 10 mg twice daily is not recommended for treatment of PsA [see Dosage and Administration (2.3)].
Clinical Response
At Month 3, patients treated with XELJANZ 5 mg twice daily had higher (p≤0.05) response rates versus placebo for ACR20, ACR50, and ACR70 in Study PsA-I and for ACR20 and ACR50 in Study PsA-II; ACR70 response rates were also higher for XELJANZ 5 mg twice daily versus placebo in Study PsA-II, although the differences versus placebo were not statistically significant (p>0.05) (Tables 14 and 15).
| Treatment Group | Placebo | XELJANZ 5 mg Twice Daily + Background Nonbiologic DMARD | |
|---|---|---|---|
| Patients with missing data were treated as non-responders. | |||
| |||
N‡ | 105 | 107 | |
Response Rate | Response Rate | Difference (%) | |
Month 3 | |||
ACR20 | 33% | 50% | 17.1 (4.1, 30.2) |
ACR50 | 10% | 28% | 18.5 (8.3, 28.7) |
ACR70 | 5% | 17% | 12.1 (3.9, 20.2) |
| Treatment Group | Placebo | XELJANZ 5 mg Twice Daily | |
|---|---|---|---|
| Patients with missing data were treated as non-responders. | |||
N† | 131 | 131 | |
Response Rate | Response Rate | Difference (%) | |
Month 3 | |||
ACR20 | 24% | 50% | 26.0 (14.7, 37.2) |
ACR50 | 15% | 30% | 15.3 (5.4, 25.2) |
ACR70 | 10% | 17% | 6.9 (-1.3, 15.1) |
Improvements from baseline in the ACR response criteria components for both studies are shown in Table 16.
| Nonbiologic DMARD Inadequate Responders (TNF Blocker-Naïve) | TNF Blocker Inadequate Responders | |||
|---|---|---|---|---|
| Study PsA-I*,† | Study PsA-II* | |||
| ||||
Treatment Group | Placebo | XELJANZ | Placebo | XELJANZ |
N at Baseline | 105 | 107 | 131 | 131 |
ACR Component‡ | ||||
Number of tender/painful joints (0–68) | ||||
Baseline | 20.6 | 20.5 | 19.8 | 20.5 |
Month 3 | 14.6 | 12.2 | 15.1 | 11.5 |
Number of swollen joints (0–66) | ||||
Baseline | 11.5 | 12.9 | 10.5 | 12.1 |
Month 3 | 7.1 | 6.3 | 7.7 | 4.8 |
Patient assessment of arthritis pain§ | ||||
Baseline | 53.2 | 55.7 | 54.9 | 56.4 |
Month 3 | 44.7 | 34.7 | 48.0 | 36.1 |
Patient global assessment of arthritis§ | ||||
Baseline | 53.9 | 54.7 | 55.8 | 57.4 |
Month 3 | 44.4 | 35.5 | 49.2 | 36.9 |
HAQ-DI¶ | ||||
Baseline | 1.11 | 1.16 | 1.25 | 1.26 |
Month 3 | 0.95 | 0.81 | 1.09 | 0.88 |
Physician's Global Assessment of Arthritis§ | ||||
Baseline | 53.8 | 54.6 | 53.7 | 53.5 |
Month 3 | 35.4 | 29.5 | 36.4 | 27.0 |
CRP (mg/L) | ||||
Baseline | 10.4 | 10.5 | 12.1 | 13.8 |
Month 3 | 8.6 | 4.0 | 11.4 | 7.7 |
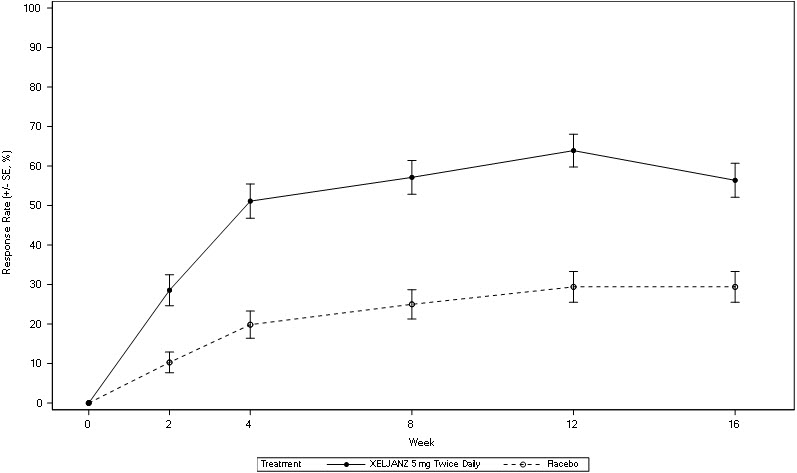
The percentage of ACR20 responders by visit for Study PsA-I is shown in Figure 5. Similar responses were observed in Study PsA-II. In both studies, improvement in ACR20 response on XELJANZ was observed at the first visit after baseline (Week 2).
| BID = twice daily; SE = standard error. |
| Patients with missing data were treated as non-responders. |
|
 |
In patients with active PsA evidence of benefit in enthesitis and dactylitis was observed with XELJANZ treatment.
Physical Function
Improvement in physical functioning was measured by the HAQ-DI. Patients receiving XELJANZ 5 mg twice daily demonstrated significantly greater improvement (p ≤0.05) from baseline in physical functioning compared to placebo at Month 3 (Table 17).
| Least Squares Mean Change from Baseline In HAQ-DI at Month 3 | ||||
|---|---|---|---|---|
| Nonbiologic DMARD Inadequate Responders* (TNF Blocker-Naïve) | TNF Blocker Inadequate Responders† | |||
| Study PsA-I‡,§ | Study PsA-II‡ | |||
| ||||
Treatment Group | Placebo | XELJANZ 5 mg | Placebo | XELJANZ 5 mg |
N¶ | 104 | 107 | 131 | 129 |
LSM Change from Baseline | -0.18 | -0.35 | -0.14 | -0.39 |
Difference from Placebo (95% CI) | - | -0.17 | - | -0.25 |
In Study PsA-I, the HAQ-DI responder rate (response defined as having improvement from baseline of ≥0.35) at Month 3 was 53% in patients receiving XELJANZ 5 mg twice daily and 31% in patients receiving placebo. Similar responses were observed in Study PsA-II.
Other Health-Related Outcomes
General health status was assessed by the Short Form health survey (SF-36). In Studies PsA-I and PsA-II, patients receiving XELJANZ 5 mg twice daily had greater improvement from baseline compared to placebo in Physical Component Summary (PCS) score, but not in Mental Component Summary (MCS) score at Month 3. Patients receiving XELJANZ 5 mg twice daily reported consistently greater improvement relative to placebo in the domains of Physical Functioning, Bodily Pain, Vitality, and Social Functioning, but not in Role-Physical, General Health, Role-Emotional, or Mental Health.
14.3 Clinical Studies in Ankylosing Spondylitis
The ankylosing spondylitis (AS) clinical development program with XELJANZ tablets (referred to as “XELJANZ” in this subsection of labeling) included one placebo-controlled trial (Study AS-I) in adults with active AS. Patients had active disease as defined by both Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and back pain score (BASDAI question 2) of greater or equal to 4 despite non-steroidal anti-inflammatory drug (NSAID), corticosteroid or disease modifying anti-rheumatic drug (DMARD) therapy.
Trial Design
Study AS-I was a randomized, double-blind, placebo-controlled, 48-week clinical trial in 269 adult patients who had an inadequate response (inadequate clinical response or intolerance) to at least 2 NSAIDs. Although Study AS-I included some patients who are TNF blocker-naïve, XELJANZ and XELJANZ XR are not approved for use in TNF blocker-naïve patients [see Indications and Usage (1.3)]. Patients were randomized and treated with XELJANZ 5 mg twice daily or placebo for 16 weeks of blinded treatment and then all received treatment of XELJANZ 5 mg twice daily for additional 32 weeks. The primary endpoint was to evaluate the proportion of patients who achieved an ASAS20 response at Week 16.
Approximately 7% and 21% of patients used concomitant methotrexate or sulfasalazine, respectively from baseline to Week 16. Twenty-two percent of patients had an inadequate response to 1 or 2 TNF blockers.
Clinical Response
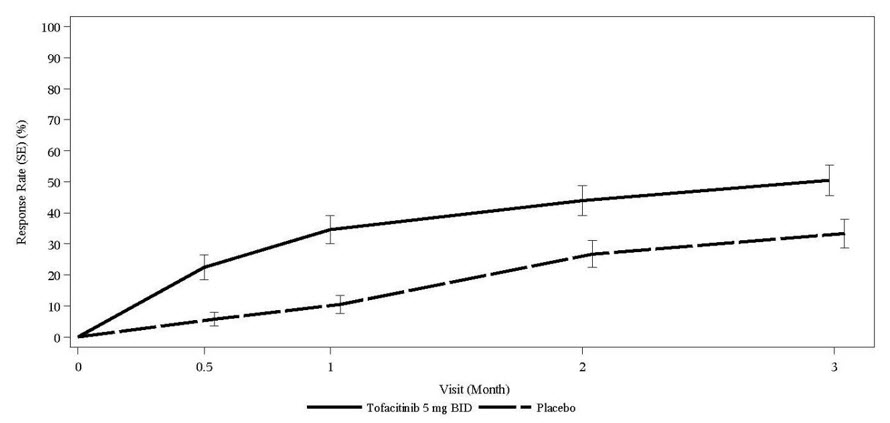
Patients treated with XELJANZ 5 mg twice daily achieved greater improvements in ASAS20 and ASAS40 responses compared to patients treated with placebo at Week 16 (Table 18). Consistent results were observed in the subgroup of patients who had an inadequate response to TNF blockers for both the ASAS20 (primary endpoint) and ASAS40 (secondary endpoint) at Week 16 (Table 18).
| Placebo | XELJANZ 5 mg Twice Daily | Difference from Placebo (95% CI) | |
|---|---|---|---|
| Abbreviations: CI = confidence interval; TNFi-IR = tumor necrosis factor inhibitor inadequate response. | |||
All patients (N) | N=136 | N=133 | |
ASAS20 response*, % | 29 | 56 | 27 (16, 38)† |
ASAS40 response*, % | 13 | 41 | 28 (18, 38)† |
TNFi-IR patients (N) | N=30 | N=29 | |
ASAS20 response, % | 17 | 41 | 25 (2, 47) |
ASAS40 response, % | 7 | 28 | 21 (2, 39) |
The improvements in the components of the ASAS response and other measures of disease activity were greater in the XELJANZ 5 mg twice daily group compared to the placebo group as shown in Table 19.
| Placebo (N=136) | XELJANZ 5 mg Twice Daily (N=133) | ||||
|---|---|---|---|---|---|
| Baseline (mean) | Week 16 (LSM change from Baseline)* | Baseline (mean) | Week 16 (LSM change from Baseline)* | Difference from Placebo (95% CI)* | |
| LSM = least squares mean. | |||||
| |||||
ASAS Components | |||||
7.0 | -1.0 | 6.9 | -2.5 | -1.5 | |
6.9 | -1.1 | 6.9 | -2.6 | -1.5 | |
5.9 | -0.8 | 5.8 | -2.0 | -1.2 | |
6.8 | -1.1 | 6.6 | -2.8 | -1.7 | |
BASDAI ScoreÞ | 6.5 | -1.2 | 6.4 | -2.6 | -1.4 |
4.4 | -0.1 | 4.5 | -0.6 | -0.5 | |
1.8 | -0.1 | 1.6 | -1.1 | -0.9 | |
The percentage of patients with active AS who achieved ASAS20 response by visit is shown in Figure 6.
Figure 6: Percentage of ASAS20 Responders Over Time Up to Week 16 in Patients with Active AS in Study AS-I
SE = standard error.
Patients with missing data were treated as non-responders.
14.4 Clinical Studies in Polyarticular Course Juvenile Idiopathic Arthritis
The efficacy of XELJANZ (tablets and oral solution) for pcJIA was assessed in Study pcJIA-I (NCT02592434). This was a 44-week, two-part study (that consisted of an 18-week, open-label, run-in phase, followed by a 26-week double-blind, placebo-controlled, randomized withdrawal phase) in pediatric patients 2 years to 17 years of age with active rheumatoid factor (RF) negative polyarthritis, RF positive polyarthritis, extended oligoarthritis, and systemic JIA without systemic manifestations who had an inadequate response or intolerance to at least one DMARD which could have included MTX or biologic agents. This study also included patients ages 2 years to 17 years of age with active juvenile psoriatic arthritis (jPsA) and enthesitis-related arthritis (ERA) who had an inadequate response to NSAIDs. Although the clinical studies included some patients who are TNF blocker-naïve, XELJANZ is not approved for use in TNF blocker-naïve patients [see Indications and Usage (1.4)].
Patients received XELJANZ (dosed at 5 mg twice daily or body weight-based equivalent twice daily) for 18 weeks (run-in phase) followed by randomization to either XELJANZ (dosed at 5 mg twice daily or body weight-based equivalent twice daily) or placebo for 26 weeks (double blind phase). Only patients who achieved at least a JIA ACR30 response at the end of the run-in phase were randomized (1:1) to the double-blind phase. Treatment with a stable dose of MTX was permitted but was not required during the study. Concurrent use of biologics or DMARDs other than MTX was not permitted in the study.
Baseline Disease Characteristics
A total of 225 pediatric patients with JIA (56 male and 169 female) with active polyarthritis were enrolled in the run-in phase including RF negative (104), RF positive (39), extended oligoarthritis (28), systemic JIA without systemic manifestations (13), jPsA (20), and ERA (21). Patients had a mean (SD) disease duration of 3.8 ± 3.5 years, and a mean (SD) number of active joints of 12.2 ± 8.1.
Efficacy Results
Of the 225 patients, 173 (77%) patients achieved JIA ACR30 response at Week 18 and were randomized into the double-blind phase to either XELJANZ (n=88) or placebo (n=85). At the conclusion of the 18-week, open-label, run-in phase, pediatric ACR 30/50/70 responses were 77%, 70%, and 49%, respectively.
In both the run-in and double-blind phases, approximately one-third of the patients were taking concomitant oral corticosteroids, and approximately two-thirds were taking concomitant MTX.
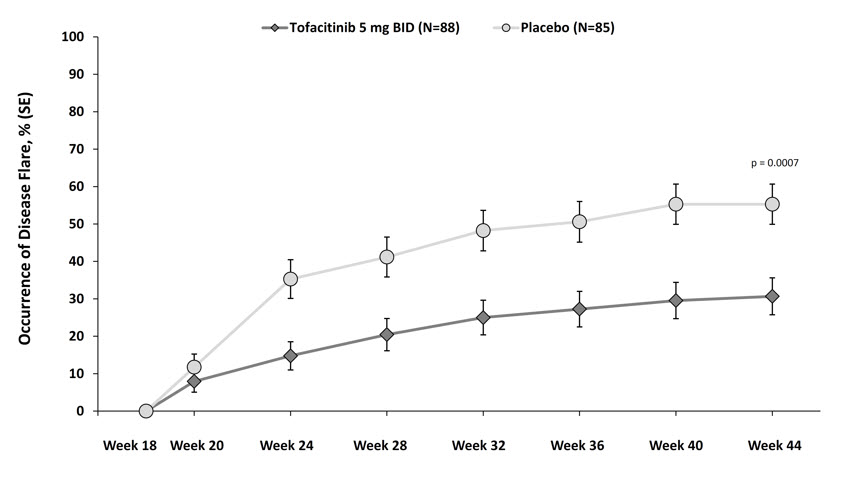
The primary endpoint was the occurrence of disease flare at Week 44 relative to the double-blind phase baseline at Week 18. Disease flare was defined (according to Pediatric Rheumatology Collaborative Study Group (PRCSG)/Pediatric Rheumatology International Trials Organization (PRINTO) Disease Flare criteria) as worsening of ≥30% in 3 or more of the 6 JIA core response variables with no more than 1 of the remaining JIA core response variables improving by ≥30%.
XELJANZ-treated patients experienced significantly fewer disease flares at Week 44 compared to placebo-treated patients (31% [27/88] vs. 55% [47/85]; difference in proportions -25% [95% CI: -39%, -10%]; p=0.0007). The occurrence of disease flare by visit in Study pcJIA-I is shown in Figure 7.
14.5 Clinical Studies in Ulcerative Colitis
The efficacy of XELJANZ tablets (referred to as “XELJANZ” in this subsection of labeling) was evaluated in two 12-week induction studies (UC-I and UC-II), a 52-week maintenance study (UC-III), and a long-term extension study (UC-IV).
Induction Trials (Study UC-I [NCT01465763] and Study UC-II [NCT01458951])
In two identical induction trials (UC-I and UC-II), 1139 adult patients were randomized (598 and 541 patients, respectively) to XELJANZ 10 mg twice daily or placebo with a 4:1 treatment allocation ratio. These trials included adult patients with moderately to severely active UC (total Mayo score of 6 to 12, with an endoscopy subscore of at least 2, and rectal bleeding subscore of at least 1) and who had failed or were intolerant to at least 1 of the following treatments: oral or intravenous corticosteroids, azathioprine, 6-MP or TNF blocker. XELJANZ and XELJANZ XR are indicated only for use in patients who have had inadequate response or intolerance to one or more TNF blockers [see Indications and Usage (1.5)].
The disease activity was assessed by Mayo scoring index (0 to 12) which consists of four subscores (0 to 3 for each subscore): stool frequency, rectal bleeding, findings on endoscopy, and physician global assessment. An endoscopy subscore of 2 was defined by marked erythema, absent vascular pattern, any friability, and erosions; an endoscopy subscore of 3 was defined by spontaneous bleeding and ulceration.
Patients were permitted to use stable doses of oral aminosalicylates and corticosteroids (prednisone daily dose up to 25 mg equivalent). Concomitant immunosuppressants (oral immunomodulators or biologic therapies) were not permitted for UC patients during these studies.
A total of 52%, 73% and 72% of patients had previously failed or were intolerant to TNF blockers (51% in Study UC-1 and 52% in Study UC-II), corticosteroids (75% in Study UC-I and 71% in Study UC-II), and/or immunosuppressants (74% in Study UC-I and 70% in Study UC-II), respectively.
Oral corticosteroids were received as concomitant treatment for UC by 47% of patients (45% in Study UC-I and 48% in Study UC-II) and 71% received concomitant aminosalicylates as treatment for UC (71% in Study UC-I, and 72% in Study UC-II). The baseline clinical characteristics were generally similar between the XELJANZ-treated patients and placebo-treated patients.
The primary endpoint of Study UC-I and Study UC-II was the proportion of patients in remission at Week 8, and the key secondary endpoint was the proportion of patients with improvement of endoscopic appearance of the mucosa at Week 8.
The efficacy results of Study UC-I and Study UC-II based on the centrally read endoscopy results are shown in Table 20.
| CI = Confidence interval; N = number of patients in the analysis set; TNF = tumor necrosis factor | |||
| |||
Study UC‑I | |||
Endpoint | Placebo | XELJANZ 10 mg Twice Daily | Treatment Difference versus Placebo (95% CI) |
Remission at Week 8* | |||
Total Population | N=122 8% | N=476 18% | 10%† (4.3, 16.3) |
With Prior TNF Blocker Failure‡ | N=64 2% | N=243 11% | |
N=58 16% | N=233 26% | ||
Improvement of Endoscopic Appearance of the Mucosa at Week 8# | |||
Total Population | N=122 16% | N=476 31% | 16%Þ (8.1, 23.4) |
With Prior TNF Blocker Failure‡ | N=64 6% | N=243 23% | |
N=58 26% | N=233 40% | ||
Study UC-II | |||
Endpoint | Placebo | XELJANZ 10 mg Twice Daily | Treatment Difference (95% CI) |
Remission at Week 8* | |||
Total Population | N=112 4% | N=429 17% | 13%Þ (8.1, 17.9) |
With Prior TNF Blocker Failure‡ | N=60 0% | N=222 12% | |
N=52 8% | N=207 22% | ||
Improvement of Endoscopic Appearance of the Mucosa at Week 8# | |||
Total Population | N=112 12% | N=429 28% | 17%Þ (9.5, 24.1) |
With Prior TNF Blocker Failure‡ | N=60 7% | N=222 22% | |
N=52 17% | N=207 36% | ||
Clinical Response at Week 8
Clinical response was defined as a decrease from baseline in Mayo score of ≥3 points and ≥30%, with an accompanying decrease in the subscore for rectal bleeding of ≥1 point or absolute subscore for rectal bleeding of 0 or 1.
Clinical response was observed in 60% of patients treated with XELJANZ 10 mg twice daily compared to 33% of patients treated with placebo in Study UC-I and 55% compared to 29% in Study UC-II.
Normalization of the Endoscopic Appearance of the Mucosa at Week 8
Normalization of endoscopic appearance of the mucosa was defined as a Mayo endoscopic subscore of 0 and was observed in 7% of patients treated with XELJANZ 10 mg twice daily compared to 2% of treated with placebo in both Studies UC-I and UC-II.
Maintenance Trial (Study UC-III [NCT01458574])
A total of 593 adult patients who completed the induction trials (UC-I or UC-II) and achieved clinical response were re-randomized with 1:1:1 treatment allocation ratio to XELJANZ 5 mg twice daily, XELJANZ 10 mg twice daily, or placebo for 52 weeks in Study UC-III. XELJANZ 5 mg twice daily is the recommended dosage for maintenance therapy; limit use of XELJANZ 10 mg twice daily beyond induction to those with loss of response and should be used for the shortest duration [see Dosage and Administration (2.5)]. As in the induction trials, patients were permitted to use stable doses of oral aminosalicylates; however, corticosteroid tapering was required upon entrance into this study for patients who were receiving corticosteroids at baseline. Concomitant immunosuppressants (oral immunomodulators or biologic therapies) were not permitted.
At baseline of Study UC-III:
- •
- 179 (30%) patients were in remission
- •
- 289 (49%) patients were receiving oral corticosteroids
- •
- 265 (45%), 445 (75%), and 413 (70%) patients had previously failed or were intolerant to TNF blocker therapy, corticosteroids, and immunosuppressants, respectively. XELJANZ and XELJANZ XR are indicated only for use in patients who have had inadequate response or intolerance to one or more TNF blockers [see Indications and Usage (1.5)].
In Study UC-III, the primary endpoint was the proportion of patients in remission at Week 52. There were two key secondary endpoints: the proportion of patients with improvement of endoscopic appearance at Week 52, and the proportion of patients with sustained corticosteroid-free remission at both Week 24 and Week 52 among patients in remission at baseline of Study UC-III.
The efficacy results of Study UC-III based on the centrally read endoscopy results are summarized in Table 21.
| Treatment Difference versus Placebo (95% CI) | |||||
|---|---|---|---|---|---|
| Endpoint | Placebo | XELJANZ 5 mg Twice Daily | XELJANZ 10 mg Twice Daily | XELJANZ 5 mg Twice Daily | XELJANZ 10 mg Twice Daily |
| CI = Confidence interval; N = number of patients in the analysis set; TNF = tumor necrosis factor. | |||||
| |||||
Remission at Week 52* | |||||
Total Population | N=198 | N=198 | N=197 | 23%† | 30%† |
11% | 34% | 41% | |||
With Prior TNF Blocker Failure‡ | N=89 | N=83 | N=93 | ||
11% | 24% | 37% | |||
N=109 | N=115 | N=104 | |||
11% | 42% | 44% | |||
Improvement of endoscopic appearance of the mucosa at Week 52# | |||||
Total Population | N=198 | N=198 | N=197 | 24%† | 33%† |
13% | 37% | 46% | |||
With Prior TNF Blocker Failure‡ | N=89 | N=83 | N=93 | ||
12% | 30% | 40% | |||
N=109 | N=115 | N=104 | |||
14% | 43% | 51% | |||
Sustained corticosteroid-free remission at both Week 24 and Week 52 among patients in remission at baselineÞ | |||||
Total Population | N=59 | N=65 | N=55 | 30%† | 42%† |
5% | 35% | 47% | |||
With Prior TNF Blocker Failure‡ | N=21 | N=18 | N=18 | ||
5% | 22% | 39% | |||
N=38 | N=47 | N=37 | |||
5% | 40% | 51% | |||
Maintenance of Clinical Response
Maintenance of clinical response was defined as the proportion of patients who met the definition of clinical response (defined as a decrease from the induction study (UC-I, UC-II) baseline Mayo score of ≥3 points and ≥30%, with an accompanying decrease in the rectal bleeding subscore of ≥1 point or rectal bleeding subscore of 0 or 1) at both Baseline and Week 52 of Study UC-III.
Maintenance of clinical response was observed in 20% in the placebo group, 52% in the XELJANZ 5 mg twice daily group, and 62% in the XELJANZ 10 mg twice daily group.
Maintenance of Remission (Among Patients in Remission at Baseline)
In the 179 patients who were in remission at baseline of Study UC-III (N = 59 for placebo, N = 65 for XELJANZ 5 mg twice daily, N = 55 for XELJANZ 10 mg twice daily), 10% in the placebo group, 46% in the XELJANZ 5 mg twice daily group and 56% in the XELJANZ 10 mg twice daily group maintained remission at Week 52.
Normalization of the Endoscopic Appearance of the Mucosa
Normalization of endoscopic appearance of the mucosa was defined as a Mayo endoscopic subscore of 0 and was observed at Week 52 in 15% of patients in the XELJANZ 5 mg twice daily group and 17% of patients in the XELJANZ 10 mg twice daily group compared to 4% of placebo patients.
Open-label Extension Study (Study UC-IV [NCT01470612])
In Study UC-IV, 914 patients were treated of which 156 received XELJANZ 5 mg twice daily and 758 received XELJANZ 10 mg twice daily.
Of the 905 patients who were assigned to XELJANZ 10 mg twice daily in the 8-week induction studies (Study UC-I or Study UC-II), 322 patients completed the induction studies but did not achieve clinical response. Of these 322 patients, 291 continued to receive XELJANZ 10 mg twice daily (unblinded) and had available data after an additional 8 weeks in Study UC-IV. After 8 additional weeks (a total of 16 weeks treatment), 148 patients achieved clinical response, and 25 patients achieved remission (based on central endoscopy read). Among those 143 patients who achieved clinical response by 16 weeks and had available data at Week 52, 66 patients achieved remission (based on local endoscopy read) after continued treatment with XELJANZ 10 mg twice daily for 52 weeks.
14.6 Safety Study in Adults with Rheumatoid Arthritis (XELJANZ Versus TNF-blocker)
A randomized open-label trial (RA Safety Study 1; NCT02092467) was conducted to evaluate safety with XELJANZ tablets (referred to as “XELJANZ” in this subsection of labeling) at two doses, 5 mg twice daily (N=1455) and 10 mg twice daily (N=1456), versus the TNF-blocker control (N=1451) in RA patients 50 years of age and older with at least one cardiovascular risk factor. The co-primary endpoints were adjudicated MACE (defined as cardiovascular death, non-fatal MI, and non-fatal stroke) and adjudicated malignancy (excluding non-melanoma skin cancer). The study was designed to exclude a prespecified risk margin of 1.8 for the hazard ratio of combined XELJANZ regimens versus the TNF-blocker control for each co-primary endpoint. An independent committee conducted a blinded evaluation of the co-primary endpoints according to predefined criteria (adjudication). The study was event-driven and patients were followed until a sufficient number of primary outcome events accrued. Other endpoints included mortality, serious infections, and thromboembolic events. The median on-study follow-up time was 4 years.
The mean age of the population was 61 years (range: 50 to 88 years). Most patients were female (78%) and Caucasian (77%). Patients had a diagnosis of RA for a mean of 10 years, and a median swollen and tender joint count of 11 and 15 respectively. Cardiovascular risk factors included cigarette smoking (current or past) (48%), hypertension (66%), high density lipoprotein <40 mg/dL (12%), diabetes mellitus (17%), family history of premature coronary heart disease (15%), extra-articular disease associated with RA (37%), and history of coronary artery disease (11%).
The non-inferiority criterion was not met for the primary comparison of the combined XELJANZ dosages to TNF blockers since the upper limit of the 95% CI exceeded the pre-specified non-inferiority criterion of 1.8 (for MACE, the upper limit of the 95% CI was 1.94; for malignancies excluding NMSC, the upper limit of the 95% CI was 2.09).
Table 22 shows the study results for each of the co-primary endpoints, and other endpoints. There was an increased risk of death, MACE, malignancies, serious infections, and thromboembolic events associated with both dosages of XELJANZ.
| Endpoint | TNF Blocker N=1451 PY=5468 | XELJANZ 5 mg Twice Daily N=1455 PY=5490 | XELJANZ 10 mg Twice Daily N=1456 PY=5298 |
|---|---|---|---|
| Note: XELJANZ 10 mg twice daily was discontinued by the Data Monitoring Committee due to safety concerns, and ongoing patients switched from XELJANZ 10 mg to XELJANZ 5 mg. The column “XELJANZ 10 mg Twice Daily” includes all events and follow-up for patients randomized to XELJANZ 10 mg twice daily. A XELJANZ (refers to tablets and oral solution) 10 mg twice daily (or a XELJANZ XR 22 mg once daily) dosage is not recommended for the treatment of RA, PsA, AS, or pcJIA [see Dosage and Administration (2.3)]. N indicates number of patients; n indicates number of patients with events. IR indicates incidence rate per 100 person-year (PY). NMSC: Non-melanoma Skin Cancer; MACE: Major Adverse Cardiac Events; HR: Hazard Ratio; DVT: Deep Vein Thrombosis; PE: Pulmonary Embolism; VTE: Venous Thromboembolism, first occurrence of a VTE, defined as the composite of adjudicated DVT and adjudicated PE; ATE: Arterial Thromboembolism; TE: Thromboembolism, first occurrence of a TE, defined as the composite of adjudicated VTE and unadjudicated ATE. | |||
| |||
MACE, n [IR] | 43 [0.79] | 50 [0.91] | 59 [1.11] |
HR (95% CI)* | 1.16 (0.77, 1.74) | 1.41 (0.95, 2.10) | |
MI,† n [IR] | 11 [0.20] | 20 [0.36] | 21 [0.39] |
HR (95% CI)* | 1.81 (0.87, 3.79) | 1.97 (0.95, 4.09) | |
Stroke,† n [IR] | 20 [0.36] | 18 [0.33] | 21 [0.39] |
HR (95% CI)* | 0.89 (0.47, 1.69) | 1.08 (0.59, 2.00) | |
Cardiovascular Death, n [IR] | 15 [0.27] | 18 [0.32] | 25 [0.47] |
HR (95% CI)* | 1.20 (0.60, 2.37) | 1.71 (0.90, 3.24) | |
Malignancies Excl. NMSC, n [IR] | 42 [0.77] | 62 [1.13] | 60 [1.13] |
HR (95% CI)* | |||
Malignancies Excl. NMSC | 25 [0.99] | 41 [1.53] | 48 [1.91] |
HR (95% CI)* | 1.55 (0.94, 2.55) | 1.94 (1.19, 3.14) | |
All Death | 38 [0.69] | 49 [0.88] | 66 [1.23] |
HR (95% CI)* | 1.29 (0.84, 1.96) | 1.79 (1.20, 2.66) | |
Serious Infections | 133 [2.52] | 155 [2.95] | 184 [3.65] |
HR (95% CI)* | 1.17 (0.93, 1.47) | 1.44 (1.15, 1.80) | |
DVT | 9 [0.16] | 12 [0.22] | 15 [0.28] |
HR (95% CI)* | 1.33 (0.56, 3.15) | 1.72 (0.75, 3.92) | |
PE | 3 [0.05] | 10 [0.18] | 26 [0.49] |
HR (95% CI)* | 3.32 (0.91, 12.08) | 8.95 (2.71, 29.56) | |
VTE | 12 [0.22] | 18 [0.33] | 36 [0.68] |
HR (95% CI)* | 1.50 (0.72, 3.10) | 3.10 (1.61, 5.96) | |
ATE | 45 [0.83] | 51 [0.93] | 55 [1.04] |
HR (95% CI)* | 1.13 (0.76, 1.69) | 1.26 (0.85, 1.87) | |
TE | 56 [1.03] | 67 [1.23] | 86 [1.65] |
HR (95% CI)* | 1.19 (0.84, 1.70) | 1.60 (1.14, 2.23) | |
Lymphomas and lung cancers, which are a subset of all malignancies in RA Safety Study 1, were observed at a higher rate in patients treated with XELJANZ 5 mg twice a day and XELJANZ 10 mg twice a day compared to those treated with TNF blockers. Lymphoma was reported for 4 patients who received XELJANZ 5 mg twice a day, 6 patients who received XELJANZ 10 mg twice a day, and 1 patient who received TNF blockers (Incidence Rate [IR] of 0.07, 0.11, and 0.02 per 100 patient-years, respectively). Among current and past smokers, lung cancer was reported for 13 patients who received XELJANZ 5 mg twice a day, 15 patients who received XELJANZ 10 mg twice a day, and 7 patients who received TNF blockers (IR of 0.48, 0.59, and 0.27 per 100 patient-years, respectively).
Given these increased risks, XELJANZ (tablets and oral solution) 10 mg twice daily (or XELJANZ XR (extended-release tablets) 22 mg once daily) dosages are not recommended for the treatment of RA, PsA, AS or pcJIA [see Dosage and Administration (2.3, 2.4)].
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.