(tofacitinib)
Health Professional Information
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tofacitinib is a Janus kinase (JAK) inhibitor. JAKs are intracellular enzymes which transmit signals arising from cytokine or growth factor-receptor interactions on the cellular membrane to influence cellular processes of hematopoiesis and immune cell function. Within the signaling pathway, JAKs phosphorylate and activate Signal Transducers and Activators of Transcription (STATs) which modulate intracellular activity including gene expression. Tofacitinib modulates the signaling pathway at the point of JAKs, preventing the phosphorylation and activation of STATs. JAK enzymes transmit cytokine signaling through pairing of JAKs (e.g., JAK1/JAK3, JAK1/JAK2, JAK1/TyK2, JAK2/JAK2). Tofacitinib inhibited the in vitro activities of JAK1/JAK2, JAK1/JAK3, and JAK2/JAK2 combinations with IC50 of 406, 56, and 1377 nM, respectively. However, the relevance of specific JAK combinations to therapeutic effectiveness is not known.
12.2 Pharmacodynamics
Treatment with XELJANZ was associated with dose-dependent reductions of circulating CD16/56+ natural killer cells, with estimated maximum reductions occurring at approximately 8-10 weeks after initiation of therapy. These changes generally resolved within 2-6 weeks after discontinuation of treatment. Treatment with XELJANZ was associated with dose-dependent increases in B cell counts. Changes in circulating T-lymphocyte counts and T-lymphocyte subsets (CD3+, CD4+ and CD8+) were small and inconsistent. The clinical significance of these changes is unknown.
Total serum IgG, IgM, and IgA levels after 6-month dosing in patients with rheumatoid arthritis (RA) were lower than in patients who received placebo; however, changes were small and not dose-dependent.
After treatment with XELJANZ in patients with RA, rapid decreases in serum C-reactive protein (CRP) were observed and maintained throughout dosing. Changes in CRP observed with XELJANZ treatment do not reverse fully within 2 weeks after discontinuation, indicating a longer duration of pharmacodynamic activity compared to the pharmacokinetic half-life.
Similar changes in T cells, B cells, and serum CRP have been observed in patients with active psoriatic arthritis (PsA) although reversibility was not assessed. Total serum immunoglobulins were not assessed in patients with active PsA.
12.3 Pharmacokinetics
Following oral administration of XELJANZ (tablets and oral solution), peak plasma concentrations were reached within 0.5 hour - 1 hour, elimination half-life was about 3 hours and a dose-proportional increase in systemic exposure was observed in the therapeutic dosage range. Steady state concentrations were achieved in 24-48 hours with negligible accumulation after twice daily administration.
Following oral administration of XELJANZ XR (extended-release tablets), peak plasma concentrations were reached at 4 hours and half-life was about 6 to 8 hours. Steady state concentrations were achieved within 48 hours with negligible accumulation after once daily administration.
Table 8 describes the pharmacokinetic parameters of XELJANZ and XELJANZ XR.
| PK Parameters* (CV%) | XELJANZ | XELJANZ XR | ||
|---|---|---|---|---|
| Dosing Regimen | 5 mg Twice Daily | 10 mg Twice Daily | 11 mg Once Daily | 22 mg Once Daily |
| Abbreviations: AUC24 = area under the concentration time profile from time 0 to 24 hours; Cmax = maximum plasma concentration; Cmin = minimum plasma concentration; Tmax = time to Cmax; CV = Coefficient of variation. | ||||
AUC24 (ng.hr/mL) | 263.4 (15) | 539.6 (22) | 269.0 (18) | 596.6 (19) |
Cmax (ng/mL) | 42.7 (26) | 84.7 (18) | 38.2 (15) | 83.8 (25) |
Cmin (ng/mL) | 1.41 (40) | 3.10 (54) | 1.07 (69) | 3.11 (43) |
Tmax (hours) | 1.0 | 0.8 | 4.0 | 4.0 |
Absorption
XELJANZ
The absolute oral bioavailability of XELJANZ is 74%. Coadministration of XELJANZ with a high-fat meal resulted in no changes in AUC while Cmax was reduced by 32%. In clinical trials, XELJANZ was administered without regard to meals [see Dosage and Administration (2.2)].
Distribution
After intravenous administration, the volume of distribution was 87 L. The protein binding of tofacitinib is approximately 40%. Tofacitinib binds predominantly to albumin and does not appear to bind to α1-acid glycoprotein. Tofacitinib distributes equally between red blood cells and plasma.
Metabolism and Excretion
Clearance mechanisms for tofacitinib are approximately 70% hepatic metabolism and 30% renal excretion of the parent drug. The metabolism of tofacitinib is primarily mediated by CYP3A4 with minor contribution from CYP2C19. In a human radiolabeled study, more than 65% of the total circulating radioactivity was accounted for by unchanged tofacitinib, with the remaining 35% attributed to 8 metabolites, each accounting for less than 8% of total radioactivity. The pharmacologic activity of tofacitinib is attributed to the parent molecule.
Pharmacokinetics in Patients with RA, PsA, AS, and UC
Population pharmacokinetic (PK) analyses indicated that PK characteristics were similar between patients with RA, PsA, ankylosing spondylitis, and UC. The coefficient of variation (%) in AUC of tofacitinib were generally similar across different disease patients, ranging from 22% to 34% (Table 9).
| Pharmacokinetic Parameters* Geometric Mean (CV%) | XELJANZ 5 mg Twice Daily | XELJANZ 10 mg Twice Daily | |||
|---|---|---|---|---|---|
| Rheumatoid Arthritis | Psoriatic Arthritis | Ankylosing Spondylitis | Ulcerative Colitis | Ulcerative Colitis | |
| Abbreviations: AUC0–24,ss = area under the plasma concentration-time curve over 24 hours at steady state; CV = coefficient of variation. | |||||
| |||||
AUC0–24,ss | 504 | 419 | 381 | 423 | 807 |
Specific Populations
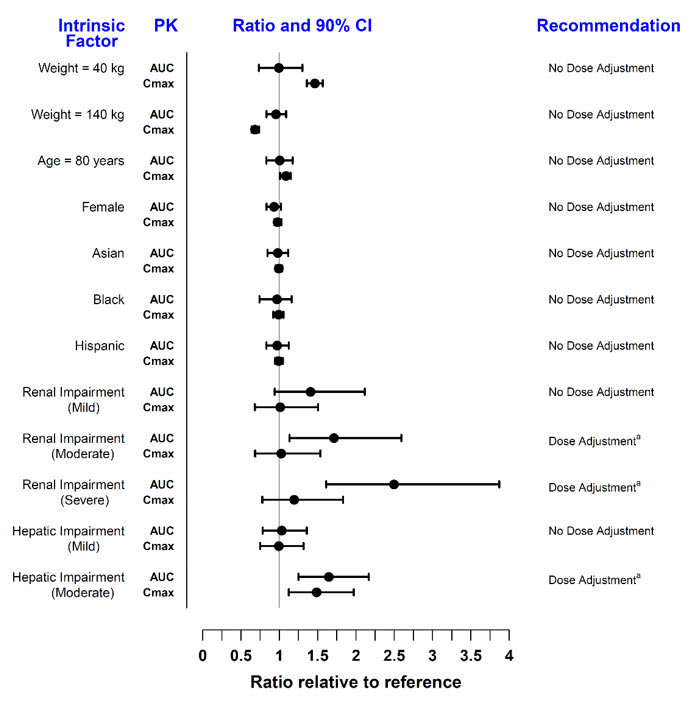
Covariate evaluation as part of population PK analyses in adult patient populations indicated no clinically relevant change in tofacitinib exposure, after accounting for differences in renal function (i.e., creatinine clearance) between patients, based on age, weight, biological sex and race (Figure 1). An approximately linear relationship between body weight and volume of distribution was observed, resulting in higher peak (Cmax) and lower trough (Cmin) concentrations in lighter patients. However, this difference is not considered to be clinically relevant.
Covariate evaluation as part of population PK analyses in pediatric patients with pcJIA, including PsA, identified body weight significantly impacting tofacitinib exposure, which supports weight-based dosing in this population. There were no identified clinically significant differences in tofacitinib exposure with different age, biological sex, racial, or pcJIA or PsA disease severity groups.
The effect of renal and hepatic impairment and other intrinsic factors on the PK of tofacitinib is shown in Figure 1.
Figure 1: Impact of Intrinsic Factors on Tofacitinib Pharmacokinetics
Note: Reference values for weight, age, biological sex, and race comparisons are 70 kg, 55 years, male, and white, respectively; reference groups for renal and hepatic impairment data are patients with normal renal and hepatic function. Renal function was estimated using creatinine clearance by Cockcroft-Gault method and hepatic function was estimated using Child-Pugh scoring method.
In patients with end-stage renal disease maintained on hemodialysis, mean AUC was approximately 40% higher compared with historical healthy subject data, consistent with approximately 30% contribution of renal clearance to the total clearance of tofacitinib [see Dosage and Administration (2.3, 2.4, 2.5) and Use in Specific Populations (8.6)].
Drug Interaction Studies
Potential for XELJANZ/XELJANZ XR to Influence the PK of Other Drugs
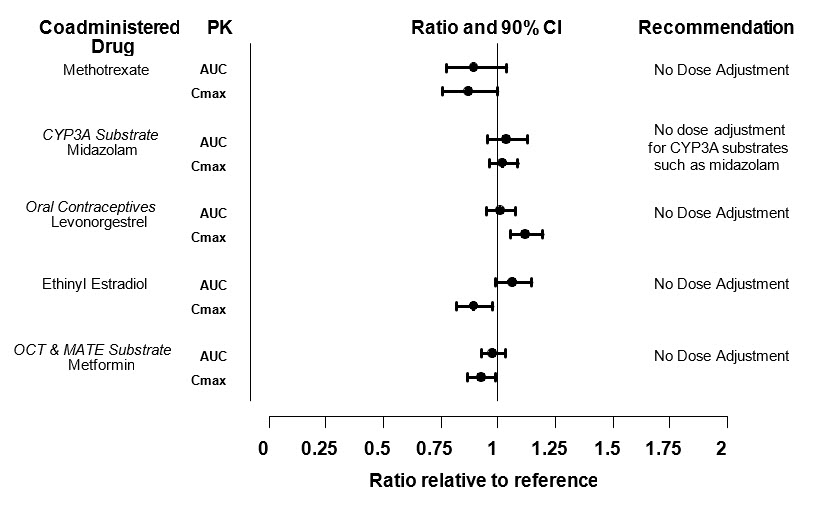
In vitro studies indicate that tofacitinib does not significantly inhibit or induce the activity of the major human drug-metabolizing CYPs (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4) at concentrations corresponding to the steady state Cmax of a 10 mg twice daily dose. These in vitro results were confirmed by a human drug interaction study showing no changes in the pharmacokinetics of midazolam, a highly sensitive CYP3A4 substrate, when concomitantly administered with XELJANZ.
In vitro studies indicate that tofacitinib does not significantly inhibit the activity of the major human drug-metabolizing uridine 5'-diphospho-glucuronosyltransferases (UGTs) [UGT1A1, UGT1A4, UGT1A6, UGT1A9, and UGT2B7] at concentrations exceeding 250 times the steady state Cmax of a 10 mg twice daily dose.
In patients with RA, the oral clearance of tofacitinib does not vary with time, indicating that tofacitinib does not normalize CYP enzyme activity in patients with RA. Therefore, concomitant use with XELJANZ/XELJANZ XR is not expected to result in clinically relevant increases in the metabolism of CYP substrates in patients with RA.
In vitro data indicate that the potential for tofacitinib to inhibit transporters such as P-glycoprotein, organic anionic or cationic transporters at therapeutic concentrations is low.
The impact of tofacitinib on the PK of other drugs for the concomitant drugs are shown in Figure 2.
Figure 2: Impact of Tofacitinib on the Pharmacokinetics of Other Drugs
Note: Reference group is administration of concomitant medication alone; OCT = Organic Cationic Transporter; MATE = Multidrug and Toxic Compound Extrusion.
Potential for Other Drugs to Influence the Pharmacokinetics of Tofacitinib
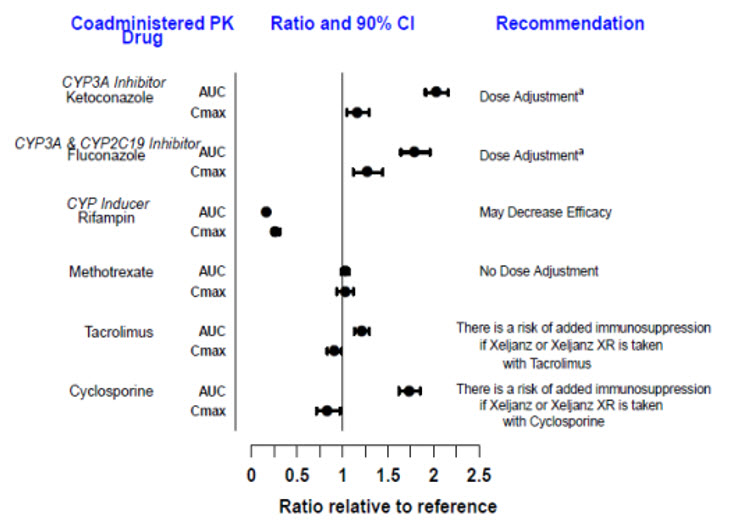
Since tofacitinib is metabolized by CYP3A4, interaction with drugs that inhibit or induce CYP3A4 is likely. Inhibitors of CYP2C19 alone or P-glycoprotein are unlikely to substantially alter the pharmacokinetics of tofacitinib (see Figure 3).
Figure 3: Impact of Other Drugs on the Pharmacokinetics of Tofacitinib
Note: Reference group is administration of XELJANZ alone.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.