(voriconazole)
Health Professional Information
Instructions For Use
INSTRUCTIONS FOR USE VFEND® (VEE-fend) (voriconazole) for oral suspension
Read this Instructions for Use before you start taking VFEND and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
Important information:
- •
- Follow your healthcare provider's instructions for the dose of VFEND to take.
- •
- Ask your healthcare provider or pharmacist if you are not sure how to take VFEND.
- •
- VFEND for oral suspension is a liquid form of VFEND. Your pharmacist will mix (reconstitute) the medicine before it is dispensed to you. If VFEND is still in powder form, do not use it. Return it to your pharmacist.
- •
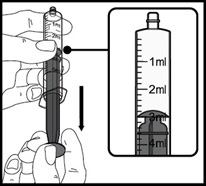
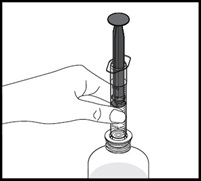
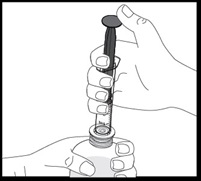
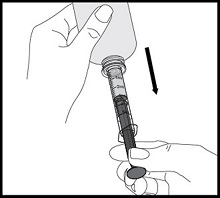
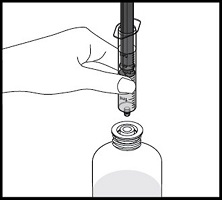
- Always use the oral dispenser provided with VFEND to make sure you measure the right amount of VFEND.
- •
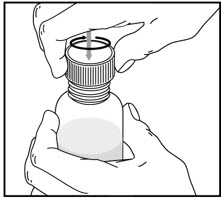
- Shake the closed bottle of mixed (reconstituted) oral suspension well for about 10 seconds before each use.
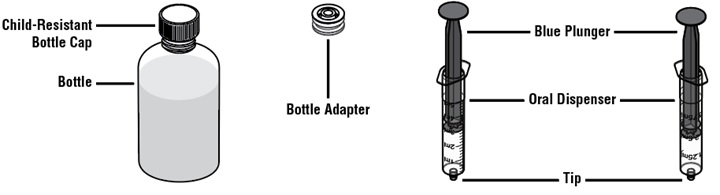
Each pack contains:
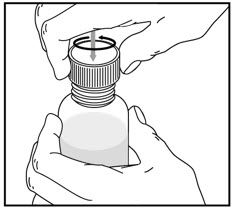
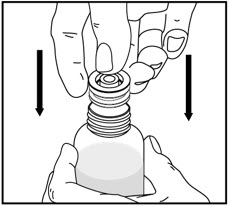
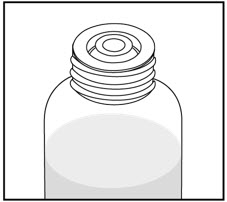
How to prepare the bottle and take VFEND:
Rinse the oral dispenser after each use.
- •
- Pull the plunger out of the oral dispenser and wash both parts with warm soapy water.
- •
- Rinse both parts with water and allow to air dry after each use.
- •
- After air drying, push the plunger back into the oral dispenser.
- •
- Store the oral dispenser with VFEND oral suspension in a clean safe place.
How should I store VFEND oral suspension?
- •
- Store VFEND oral suspension at room temperature between 59°F to 86°F (15°C to 30°C).
- •
- Do not refrigerate or freeze.
- •
- Keep the bottle cap tightly closed.
- •
- Use VFEND oral suspension within 14 days after it has been mixed (reconstituted) by the pharmacist. The pharmacist will write the expiration date on the bottle label (the expiration date of the oral suspension is 14 days from the date it was mixed (reconstituted) by the pharmacist). Throw away (discard) any unused VFEND after the expiration date.
- •
- Keep VFEND and all medicines out of the reach of children.
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
LAB-1348-6.0
Revised: 8/2024
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.