(tucatinib)
Health Professional Information
Description
11 DESCRIPTION
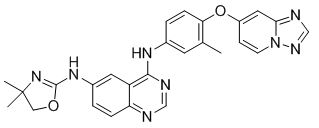
Tucatinib is a kinase inhibitor. The chemical name is (N4-(4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)-3-methylphenyl)-N6-(4,4-dimethyl-4,5-dihydrooxazol-2-yl)quinazoline-4,6-diamine. The molecular formula is C26H24N8O2 and the molecular weight is 480.52 g/mol. The chemical structure is as follows:
TUKYSA (tucatinib) is supplied as 50 mg and 150 mg film-coated tablets for oral use and contain the following inactive ingredients:
Tablet core: copovidone, crospovidone, sodium chloride, potassium chloride, sodium bicarbonate, colloidal silicon dioxide, magnesium stearate, and microcrystalline cellulose.
Coating: yellow film coat: polyvinyl alcohol, titanium dioxide, macrogol/polyethylene glycol, talc, and yellow iron oxide non-irradiated.
Each TUKYSA 50 mg tablet contains 10.10 mg (0.258 mEq) potassium and 9.21 mg (0.401 mEq) sodium.
Each TUKYSA 150 mg tablet contains 30.29 mg (0.775 mEq) potassium and 27.64 mg (1.202 mEq) sodium.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.