Health Professional Information
Description
DESCRIPTION
Testosterone Cypionate Injection, for intramuscular injection, contains testosterone cypionate which is the oil-soluble 17 (beta)- cyclopentylpropionate ester of the androgenic hormone testosterone.
Testosterone cypionate is a white or creamy white crystalline powder, odorless or nearly so and stable in air. It is insoluble in water, freely soluble in alcohol, chloroform, dioxane, ether, and soluble in vegetable oils.
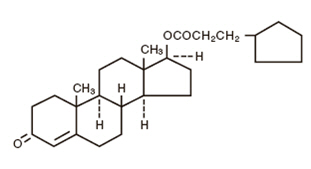
The chemical name for testosterone cypionate is androst-4-en-3-one, 17-(3-cyclopentyl-1-oxopropoxy)-, (17ß)-. Its molecular formula is C27H40O3, and the molecular weight 412.61.
The structural formula is represented below:
Testosterone Cypionate Injection is available in two strengths, 100 mg/mL and 200 mg/mL testosterone cypionate.
Each mL of the 100 mg/mL solution contains: | ||
Testosterone cypionate | 100 mg | |
Benzyl benzoate | 0.1 mL | |
Cottonseed oil | 736 mg | |
Benzyl alcohol (as preservative) | 9.45 mg | |
Each mL of the 200 mg/mL solution contains: | ||
Testosterone cypionate | 200 mg | |
Benzyl benzoate | 0.2 mL | |
Cottonseed oil | 560 mg | |
Benzyl alcohol (as preservative) | 9.45 mg | |
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.