(talazoparib)
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
14.1 Deleterious or Suspected Deleterious Germline BRCA-mutated HER2-negative Locally Advanced or Metastatic Breast Cancer
EMBRACA (NCT01945775) was an open-label study in which patients (N=431) with gBRCAm HER2-negative locally advanced or metastatic breast cancer were randomized 2:1 to receive TALZENNA 1 mg or healthcare provider's choice of chemotherapy (capecitabine, eribulin, gemcitabine, or vinorelbine) until disease progression or unacceptable toxicity. Randomization was stratified by prior lines of chemotherapy for metastatic disease (0 versus 1, 2, or 3), by triple-negative disease status [triple-negative breast cancer (TNBC) versus non-TNBC], and history of central nervous system (CNS) metastasis (yes versus no).
Patients received no more than 3 prior cytotoxic chemotherapy regimens for their metastatic or locally advanced disease. Patients were required to have received treatment with an anthracycline and/or a taxane (unless contraindicated) in the neoadjuvant, adjuvant, and/or metastatic treatment setting. First-line treatment for advanced or metastatic disease with no prior adjuvant chemotherapy was allowed if the investigator determined that 1 of the 4 chemotherapy choices in the control arm would be an appropriate treatment option for the patient. Patients with prior platinum therapy for advanced disease were required to have no evidence of disease progression during platinum therapy. No prior treatment with a PARP inhibitor was permitted. Of the 431 patients randomized in the EMBRACA study, 408 (95%) were centrally confirmed to have a deleterious or suspected deleterious gBRCAm using a clinical trial assay; out of which 354 (82%) were confirmed using the BRACAnalysis CDx®. BRCA mutation status [breast cancer susceptibility gene 1 (BRCA1)-positive or breast cancer susceptibility gene 2 (BRCA2)-positive] was similar across both treatment arms.
The median age of patients treated with TALZENNA was 46 years (range 28 to 84) and 51 years (range 24 to 89) among patients treated with chemotherapy. Among all randomized patients, 1% versus 2% were males, 67% versus 75% were White; 11% versus 11% were Asian, and 4% versus 1% were Black or African American in the TALZENNA and chemotherapy arms, respectively. Almost all patients (98%) in both arms had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Approximately 56% of patients had estrogen receptor-positive and/or progesterone receptor-positive disease; 44% of patients had triple-negative disease, and the proportions were balanced across both treatment arms. Fifteen percent (15%) of patients in the TALZENNA arm and 14% of patients in the chemotherapy arm had a history of CNS metastases. Ninety-one percent (91%) of patients in the TALZENNA arm had received prior taxane therapy, and 85% had received prior anthracycline therapy in any setting. Sixteen percent (16%) of patients in the TALZENNA arm and 21% of patients in the chemotherapy arm had received prior platinum treatment in any setting. The median number of prior cytotoxic regimens for patients with advanced breast cancer was one; 38% received no prior cytotoxic regimens for advanced or metastatic disease, 37% received one, 20% received two, and 5% received three or more prior cytotoxic regimens.
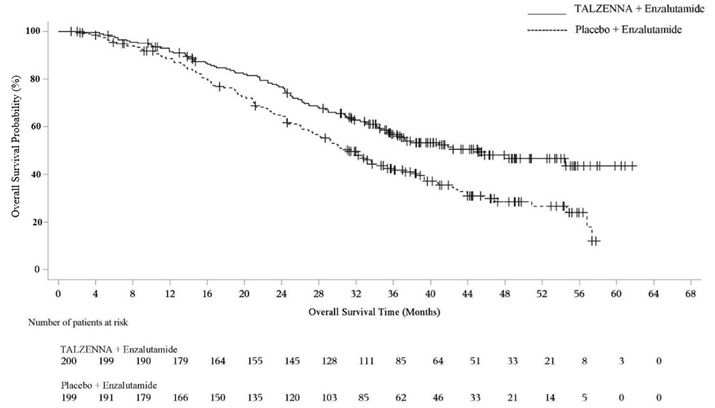
The major efficacy outcome measure was progression-free survival (PFS) evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, as assessed by blinded independent central review (BICR). A statistically significant improvement in PFS was demonstrated for TALZENNA compared to chemotherapy. A sensitivity analysis of investigator-assessed PFS was consistent with the BICR-assessed PFS results. Consistent PFS results were observed across patient subgroups defined by study stratification factors (prior lines of chemotherapy, TNBC status, and history of CNS metastases). Efficacy data from the EMBRACA study are summarized in Table 9, and the Kaplan-Meier curves for PFS are shown in Figure 1 and final overall survival (OS) in Figure 2.
| Abbreviations: BICR=blinded independent central review; CI=confidence interval; DOR=duration of response; ITT=intent-to-treat; N=number of patients; ORR=objective response rate; OS=overall survival; PFS=progression-free survival. | ||
| ||
TALZENNA | Chemotherapy | |
PFS by BICR | N=287 | N=144 |
Disease progression or deaths, n (%) | 186 (65) | 83 (58) |
Median months (95% CI) | 8.6 (7.2, 9.3) | 5.6 (4.2, 6.7) |
Hazard ratio (95% CI)* | 0.54 (0.41, 0.71) | |
p-value† | p<0.0001 | |
Patients with Measurable Disease by Investigator‡ | N=219 | N=114 |
ORR, % (95% CI)§ | 50.2 (43.4, 57.0) | 18.4 (11.8, 26.8) |
Median¶ DOR months (95% CI) | 6.4 (5.4, 9.5) | 3.9 (3.0, 7.6) |
OS | N=287 | N=144 |
Deaths, n (%) | 216 (75) | 108 (75) |
Median months (95% CI) | 19.3 (16.6, 22.5) | 19.5 (17.4, 22.4) |
Hazard ratio (95% CI)* | 0.85 (0.67, 1.07) | |
p-value† | p=0.1693 | |
Figure 1. Kaplan-Meier Curves of PFS – EMBRACA Study
| Abbreviation: PFS=progression-free survival. |
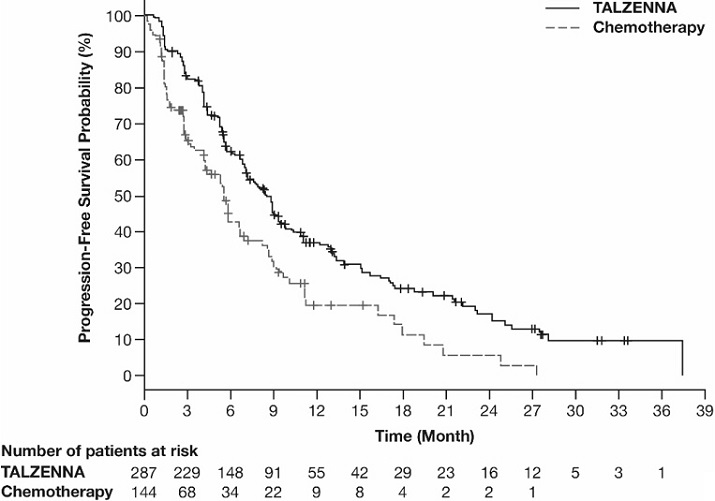 |
Figure 2. Kaplan-Meier Curves of OS – EMBRACA Study (ITT Population)
| Abbreviations: ITT=intent-to-treat; OS=overall survival. |
 |
14.2 HRR Gene-mutated mCRPC
The efficacy of TALZENNA with enzalutamide was evaluated in TALAPRO-2 (NCT03395197), a randomized, double-blind, placebo-controlled, multi-cohort trial in which 399 patients with HRR gene-mutated (HRRm) mCRPC were randomized 1:1 to receive enzalutamide 160 mg daily plus either TALZENNA 0.5 mg or placebo daily until unacceptable toxicity or disease progression. Mutation status of HRR genes was determined prospectively using solid tumor tissue or circulating tumor DNA (ctDNA)-based next generation sequencing assays. Patients were required to have a mutation in at least one of 12 genes involved directly or indirectly in the HRR pathway (ATM, ATR, BRCA1, BRCA2, CDK12, CHEK2, FANCA, MLH1, MRE11A, NBN, PALB2, or RAD51C). All patients received a GnRH analog or had prior bilateral orchiectomy and needed to have progressed on prior androgen deprivation therapy. Prior treatment with a CYP17 inhibitor or docetaxel for metastatic castration-sensitive prostate cancer (mCSPC) was permitted. Randomization was stratified by previous treatment with a CYP17 inhibitor or docetaxel (yes/no).
The median age was 70 years (range: 41 to 90); 100% were male; 68% were White, 21% Asian, 2.8% Black, 0.8% Other, 7% unknown/not reported; 12% were Hispanic/Latino; and baseline ECOG performance status was 0 (62%) or 1 (38%). Thirty-nine percent of patients had bone-only disease; 15% had visceral disease. In the mCSPC setting, 29% percent of patients had received docetaxel and 9% had received a prior CYP17 inhibitor. The most commonly mutated HRR genes (>5%), including co-occurring mutations, were: BRCA2 (34%), ATM (22%), CDK12 (19%), CHEK2 (18%), and BRCA1 (6%).
The major efficacy outcome measure was radiographic progression-free survival (rPFS) evaluated according to RECIST, version 1.1 and Prostate Cancer Working Group (PCWG3) (bone) criteria, assessed by BICR. An additional efficacy outcome measure was OS.
A statistically significant improvement in rPFS and OS were demonstrated in patients randomized to TALZENNA with enzalutamide compared with placebo with enzalutamide. Efficacy results are presented in Table 10, and Figures 3 and 4.
| Abbreviations: BICR=blinded independent central review; CI=confidence interval; CSPC=castration-sensitive prostate cancer; HRR=homologous recombination repair; mCRPC=metastatic castration-resistant prostate cancer; N=number of patients; NR=not reached. | |||
TALZENNA with Enzalutamide N=200 | Placebo with Enzalutamide N=199 | ||
Radiographic Progression-free Survival (rPFS) by BICR | |||
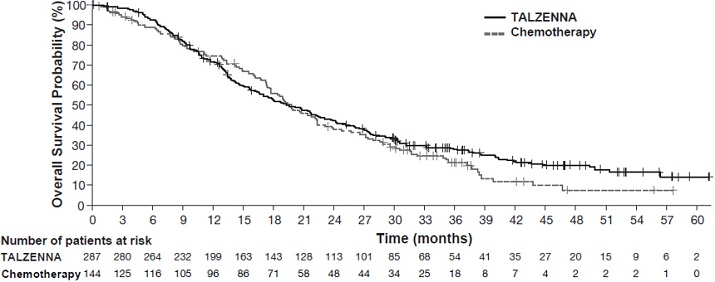
Number of events, n (%) | 66 (33) | 104 (52) | |
Median months (95% CI) | NR (21.9, NR) | 13.8 (11.0, 16.7) | |
Hazard ratio (95% CI)* | 0.45 (0.33, 0.61) | ||
p-value† | p<0.0001 | ||
| |||
Overall Survival (OS) | |||
Deaths, n (%) | 93 (47) | 126 (63) | |
Median months (95% CI) | 45.1 (35.4, NR) | 31.1 (27.3, 35.4) | |
Hazard ratio (95% CI)* | 0.62 (0.48, 0.81) | ||
p-value† | p=0.0005 | ||
Figure 3. Kaplan-Meier Curves of rPFS - TALAPRO-2 (HRR Gene-mutated mCRPC)
| Abbreviations: HRR=homologous recombination repair; mCRPC=metastatic castration-resistant prostate cancer; rPFS=radiographic progression-free survival. |
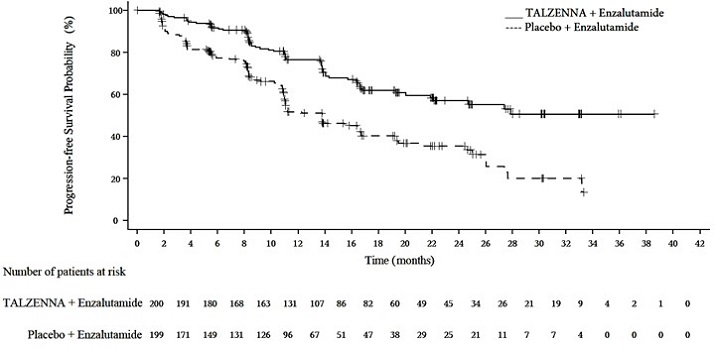 |
Figure 4. Kaplan-Meier Curves of OS – TALAPRO-2 (HRR Gene-mutated mCRPC)
Abbreviations: HRR=homologous recombination repair; mCRPC=metastatic castration-resistant prostate cancer; OS=overall survival.
Exploratory subgroup analyses of rPFS and OS for patients with BRCA-mutated (BRCAm) and non-BRCAm HRRm are presented in Table 11.
| Abbreviations: BRCAm=breast cancer susceptibility gene-mutated; CI=confidence interval; CSPC=castration-sensitive prostate cancer; HRRm=homologous recombination repair gene-mutated; NR=not reached; rPFS=radiographic progression-free survival. | ||||
BRCAm | Non-BRCAm HRRm* | |||
TALZENNA with Enzalutamide N=71 | Placebo with Enzalutamide N=84 | TALZENNA with Enzalutamide N=129 | Placebo with Enzalutamide N=115 | |
rPFS | ||||
Number of events, n (%) | 15 (21) | 54 (64) | 51 (40) | 50 (43) |
Median months (95% CI) | NR (NR, NR) | 11.0 (8.3, 11.1) | 24.7 (16.4, NR) | 16.7 (13.8, 27.7) |
Hazard ratio (95% CI) | 0.20 (0.11, 0.36) | 0.74 (0.50, 1.09) | ||
| ||||
Overall Survival (OS) | ||||
Deaths, n (%) | 30 (42) | 56 (67) | 63 (49) | 70 (61) |
Median months (95% CI) | NR (35.4, NR) | 28.5 (24.5, 34.4) | 42.4 (34.2, NR) | 32.6 (28.0, 42.2) |
Hazard ratio (95% CI)† | 0.48 (0.31, 0.75) | 0.71 (0.51, 1.00) | ||
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.