(methylprednisolone sodium succinate)
Health Professional Information
Description
DESCRIPTION
SOLU-MEDROL Sterile Powder is an anti-inflammatory glucocorticoid, which contains methylprednisolone sodium succinate as the active ingredient. Methylprednisolone sodium succinate, USP, is the sodium succinate ester of methylprednisolone, and it occurs as a white, or nearly white, odorless hygroscopic, amorphous solid. It is very soluble in water and in alcohol; it is insoluble in chloroform and is very slightly soluble in acetone.
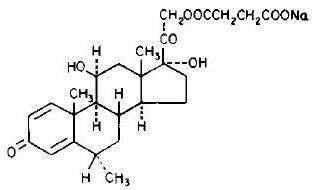
The chemical name for methylprednisolone sodium succinate is pregna-1,4-diene-3,20-dione,21-(3-carboxy-1-oxopropoxy)-11,17-dihydroxy-6-methyl-monosodium salt, (6α, 11β), and the molecular weight is 496.53. The structural formula is represented below:
Methylprednisolone sodium succinate is soluble in water; it may be administered in a small volume of diluent and is well suited for intravenous use in situations where high blood levels of methylprednisolone are required rapidly.
SOLU-MEDROL is available in preservative and preservative-free formulations:
40 mg Act-O-Vial System (Single-Dose Vial)—Each mL (when mixed) contains methylprednisolone sodium succinate equivalent to 40 mg methylprednisolone; also |
125 mg Act-O-Vial System (Single-Dose Vial)—Each 2 mL (when mixed) contains methylprednisolone sodium succinate equivalent to 125 mg methylprednisolone; also |
500 mg Act-O-Vial System (Single-Dose Vial)—Each 4 mL (when mixed) contains methylprednisolone sodium succinate equivalent to 500 mg methylprednisolone; also |
1 gram Act-O-Vial System (Single-Dose Vial)—Each 8 mL (when mixed) contains methylprednisolone sodium succinate equivalent to 1 gram methylprednisolone; also |
500 mg Vial—Each 8 mL (when mixed as directed) contains methylprednisolone sodium succinate equivalent to 500 mg methylprednisolone; also 6.4 mg monobasic sodium phosphate anhydrous; 69.6 mg dibasic sodium phosphate dried. |
1 gram Vial—Each 16 mL (when mixed as directed) contains methylprednisolone sodium succinate equivalent to 1 gram methylprednisolone; also 12.8 mg monobasic sodium phosphate anhydrous; 139.2 mg dibasic sodium phosphate dried. |
2 gram Vial—Each 30.6 mL (when mixed as directed) contains methylprednisolone sodium succinate equivalent to 2 grams methylprednisolone; also 25.6 mg monobasic sodium phosphate anhydrous; 278 mg dibasic sodium phosphate dried. |
IMPORTANT — Use only the accompanying diluent |
When necessary, the pH of each formula was adjusted with sodium hydroxide so that the pH of the reconstituted solution is within the USP specified range of 7 to 8.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.