(rituximab-pvvr)
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
14.1 Relapsed or Refractory, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
The safety and effectiveness of rituximab in relapsed, refractory CD20+ NHL were demonstrated in 3 single-arm studies enrolling 296 patients.
NHL Study 1
A multicenter, open-label, single-arm study was conducted in 166 patients with relapsed or refractory, low-grade or follicular, B-cell NHL who received 375 mg/m2 of rituximab given as an intravenous infusion weekly for 4 doses. Patients with tumor masses greater than 10 cm or with greater than 5,000 lymphocytes/µL in the peripheral blood were excluded from the study.
Results are summarized in Table 8. The median time to onset of response was 50 days. Disease-related signs and symptoms (including B symptoms) resolved in 64% (25/39) of those patients with such symptoms at study entry.
NHL Study 2
In a multicenter, single-arm study, 37 patients with relapsed or refractory, low-grade NHL received 375 mg/m2 of rituximab weekly for 8 doses. Results are summarized in Table 8.
NHL Study 3
In a multicenter, single-arm study, 60 patients received 375 mg/m2 of rituximab weekly for 4 doses. All patients had relapsed or refractory, low-grade or follicular, B-cell NHL and had achieved an objective clinical response to rituximab administered 3.8–35.6 months (median 14.5 months) prior to retreatment with rituximab. Of these 60 patients, 5 received more than one additional course of rituximab. Results are summarized in Table 8.
Bulky Disease
In pooled data from studies 1 and 3, 39 patients with bulky (single lesion greater than 10 cm in diameter) and relapsed or refractory, low-grade NHL received rituximab 375 mg/m2 weekly for 4 doses. Results are summarized in Table 8.
NHL Study 1 |
NHL Study 2 |
NHL Study 1 and |
NHL Study 3 |
|
Overall Response Rate |
48% |
57% |
36% |
38% |
Complete Response Rate |
6% |
14% |
3% |
10% |
11.2 |
13.4 |
6.9 |
15.0 |
|
(Months) [Range] |
[1.9 to 42.1+] |
[2.5 to 36.5+] |
[2.8 to 25.0+] |
[3.0 to 25.1+] |
14.2 Previously Untreated, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
The safety and effectiveness of rituximab in previously untreated, low-grade or follicular, CD20+ NHL were demonstrated in 3 randomized, controlled trials enrolling 1,662 patients.
NHL Study 4
A total of 322 patients with previously untreated follicular NHL were randomized (1:1) to receive up to eight 3-week cycles of CVP chemotherapy alone (CVP) or in combination with rituximab 375 mg/m2 on Day 1 of each cycle (R-CVP) in an open-label, multicenter study. The main outcome measure of the study was progression-free survival (PFS) defined as the time from randomization to the first of progression, relapse, or death.
Twenty-six percent of the study population was greater than 60 years of age, 99% had Stage III or IV disease, and 50% had an International Prognostic Index (IPI) score greater than or equal to 2. The results for PFS as determined by a blinded, independent assessment of progression are presented in Table 9. The point estimates may be influenced by the presence of informative censoring. The PFS results based on investigator assessment of progression were similar to those obtained by the independent review assessment.
Study Arm |
|||
R-CVP |
CVP |
||
Median PFS (years)* |
2.4 |
1.4 |
|
Hazard ratio (95% CI)† |
0.44 (0.29, 0.65) |
||
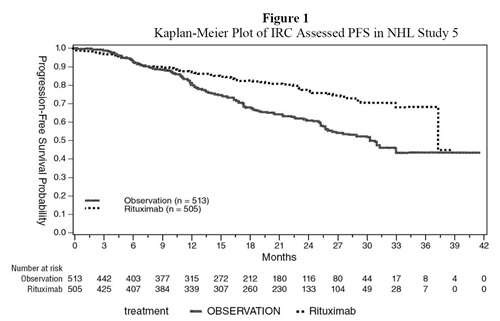
NHL Study 5
An open-label, multicenter, randomized (1:1) study was conducted in 1,018 patients with previously untreated follicular NHL who achieved a response (CR or PR) to rituximab in combination with chemotherapy. Patients were randomized to rituximab as single-agent maintenance therapy, 375 mg/m2 every 8 weeks for up to 12 doses or to observation. Rituximab was initiated at 8 weeks following completion of chemotherapy. The main outcome measure of the study was progression-free survival (PFS), defined as the time from randomization in the maintenance/observation phase to progression, relapse, or death, as determined by independent review.
Of the randomized patients, 40% were greater than or equal to 60 years of age, 70% had Stage IV disease, 96% had ECOG performance status (PS) 0–1, and 42% had FLIPI scores of 3–5. Prior to randomization to maintenance therapy, patients had received R-CHOP (75%), R-CVP (22%), or R-FCM (3%); 71% had a complete or unconfirmed complete response and 28% had a partial response.
PFS was longer in patients randomized to rituximab as single-agent maintenance therapy (HR: 0.54, 95% CI: 0.42, 0.70). The PFS results based on investigator assessment of progression were similar to those obtained by the independent review assessment.
Figure 1 |
|
NHL Study 6
A total of 322 patients with previously untreated low-grade, B-cell NHL who did not progress after 6 or 8 cycles of CVP chemotherapy were enrolled in an open-label, multicenter, randomized trial. Patients were randomized (1:1) to receive rituximab, 375 mg/m2 intravenous infusion, once weekly for 4 doses every 6 months for up to 16 doses or no further therapeutic intervention. The main outcome measure of the study was progression-free survival defined as the time from randomization to progression, relapse, or death. Thirty-seven percent of the study population was greater than 60 years of age, 99% had Stage III or IV disease, and 63% had an IPI score greater than or equal to 2.
There was a reduction in the risk of progression, relapse, or death (hazard ratio estimate in the range of 0.36 to 0.49) for patients randomized to rituximab as compared to those who received no additional treatment.
14.3 Diffuse Large B-Cell NHL (DLBCL)
The safety and effectiveness of rituximab were evaluated in three randomized, active-controlled, open-label, multicenter studies with a collective enrollment of 1,854 patients. Patients with previously untreated diffuse large B-cell NHL received rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or other anthracycline-based chemotherapy regimens.
NHL Study 7
A total of 632 patients age greater than or equal to 60 years with DLBCL (including primary mediastinal B‑cell lymphoma) were randomized in a 1:1 ratio to treatment with CHOP or R-CHOP. Patients received 6 or 8 cycles of CHOP, each cycle lasting 21 days. All patients in the R-CHOP arm received 4 doses of rituximab 375 mg/m2 on Days –7 and –3 (prior to Cycle 1) and 48–72 hours prior to Cycles 3 and 5. Patients who received 8 cycles of CHOP also received rituximab prior to Cycle 7. The main outcome measure of the study was progression‑free survival, defined as the time from randomization to the first of progression, relapse, or death. Responding patients underwent a second randomization to receive rituximab or no further therapy.
Among all enrolled patients, 62% had centrally confirmed DLBCL histology, 73% had Stage III–IV disease, 56% had IPI scores greater than or equal to 2, 86% had ECOG performance status of less than 2, 57% had elevated LDH levels, and 30% had two or more extranodal disease sites involved. Efficacy results are presented in Table 10. These results reflect a statistical approach which allows for an evaluation of rituximab administered in the induction setting that excludes any potential impact of rituximab given after the second randomization.
Analysis of results after the second randomization in NHL Study 7 demonstrates that for patients randomized to R-CHOP, additional rituximab exposure beyond induction was not associated with further improvements in progression-free survival or overall survival.
NHL Study 8
A total of 399 patients with DLBCL, age greater than or equal to 60 years, were randomized in a 1:1 ratio to receive CHOP or R-CHOP. All patients received up to eight 3-week cycles of CHOP induction; patients in the R-CHOP arm received rituximab 375 mg/m2 on Day 1 of each cycle. The main outcome measure of the study was event-free survival, defined as the time from randomization to relapse, progression, change in therapy, or death from any cause. Among all enrolled patients, 80% had Stage III or IV disease, 60% of patients had an age-adjusted IPI greater than or equal to 2, 80% had ECOG performance status scores less than 2, 66% had elevated LDH levels, and 52% had extranodal involvement in at least two sites. Efficacy results are presented in Table 10.
NHL Study 9
A total of 823 patients with DLBCL, aged 18–60 years, were randomized in a 1:1 ratio to receive an anthracycline-containing chemotherapy regimen alone or in combination with rituximab. The main outcome measure of the study was time to treatment failure, defined as time from randomization to the earliest of progressive disease, failure to achieve a complete response, relapse, or death. Among all enrolled patients, 28% had Stage III–IV disease, 100% had IPI scores of less than or equal to 1, 99% had ECOG performance status of less than 2, 29% had elevated LDH levels, 49% had bulky disease, and 34% had extranodal involvement. Efficacy results are presented in Table 10.
|
NHL Study 7 (n=632) |
NHL Study 8 (n=399) |
NHL Study 9 (n=823) |
||||
R-CHOP |
CHOP |
R-CHOP |
CHOP |
R-Chemo |
Chemo |
|
Main outcome |
Progression-free survival (years) |
Event-free survival (years) |
Time to treatment failure (years) |
|||
Median of main outcome |
3.1 |
1.6 |
2.9 |
1.1 |
NE* |
NE* |
Hazard ratio† |
0.69‡ |
0.60‡ |
0.45‡ |
|||
Overall survival at 2 years§ |
74% |
63% |
69% |
58% |
95% |
86% |
Hazard ratio† |
0.72‡ |
0.68‡ |
0.40‡ |
|||
In NHL Study 8, overall survival estimates at 5 years were 58% vs. 46% for R-CHOP and CHOP, respectively.
14.4 Ninety-Minute Infusions in Previously Untreated Follicular NHL and DLBCL
In NHL Study 10, a total of 363 patients with previously untreated follicular NHL (n=113) or DLBCL (n=250) were evaluated in a prospective, open-label, multicenter, single-arm trial for the safety of 90-minute rituximab infusions. Patients with follicular NHL received rituximab 375 mg/m2 plus CVP chemotherapy. Patients with DLBCL received rituximab 375 mg/m2 plus CHOP chemotherapy. Patients with clinically significant cardiovascular disease were excluded from the study. Patients were eligible for a 90-minute infusion at Cycle 2 if they did not experience a Grade 3–4 infusion-related adverse event with Cycle 1 and had a circulating lymphocyte count less than or equal to 5,000/mm3 before Cycle 2. All patients were pre-medicated with acetaminophen and an antihistamine and received the glucocorticoid component of their chemotherapy prior to rituximab infusion. The main outcome measure was the development of Grade 3–4 infusion-related reactions on the day of, or day after, the 90-minute infusion at Cycle 2 [see Adverse Reactions (6.1)].
Eligible patients received their Cycle 2 rituximab infusion over 90 minutes as follows: 20% of the total dose given in the first 30 minutes and the remaining 80% of the total dose given over the next 60 minutes [see Dosage and Administration (2.1)]. Patients who tolerated the 90-minute rituximab infusion at Cycle 2 continued to receive subsequent rituximab infusions at the 90-minute infusion rate for the remainder of the treatment regimen (through Cycle 6 or Cycle 8).
The incidence of Grade 3–4 infusion-related reactions at Cycle 2 was 1.1% (95% CI [0.3%, 2.8%]) among all patients, 3.5% (95% CI [1.0%, 8.8%]) for those patients treated with R-CVP, and 0.0% (95% CI [0.0%, 1.5%]) for those patients treated with R-CHOP. For Cycles 2–8, the incidence of Grade 3–4 infusion-related reactions was 2.8% (95% CI [1.3%, 5.0%]). No acute fatal infusion-related reactions were observed.
14.5 Chronic Lymphocytic Leukemia (CLL)
The safety and effectiveness of rituximab were evaluated in two randomized (1:1) multicenter open-label studies comparing FC alone or in combination with rituximab for up to 6 cycles in patients with previously untreated CLL [CLL Study 1 (n=817)] or previously treated CLL [CLL Study 2 (n=552)]. Patients received fludarabine 25 mg/m2/day and cyclophosphamide 250 mg/m2/day on days 1, 2, and 3 of each cycle, with or without rituximab. In both studies, seventy-one percent of CLL patients received 6 cycles and 90% received at least 3 cycles of rituximab-based therapy.
In CLL Study 1, 30% of patients were 65 years or older, 31% were Binet stage C, 45% had B symptoms, more than 99% had ECOG performance status (PS) 0–1, 74% were male, and 100% were White. In CLL Study 2, 44% of patients were 65 years or older, 28% had B symptoms, 82% received a prior alkylating drug, 18% received prior fludarabine, 100% had ECOG PS 0–1, 67% were male, and 98% were White.
The main outcome measure in both studies was progression-free survival (PFS), defined as the time from randomization to progression, relapse, or death, as determined by investigators (CLL Study 1) or an independent review committee (CLL Study 2). The investigator assessed results in CLL Study 2 were supportive of those obtained by the independent review committee. Efficacy results are presented in Table 11.
| ||||
|
CLL Study 1* (Previously untreated) |
CLL Study 2* (Previously treated) |
|||
|
R-FC N=408 |
FC N=409 |
R-FC N=276 |
FC N=276 |
|
Median PFS (months) |
39.8 |
31.5 |
26.7 |
21.7 |
Hazard ratio (95% CI) |
0.56 (0.43, 0.71) |
0.76 (0.6, 0.96) |
||
P value (Log-Rank test) |
<0.01 |
0.02 |
||
Response rate |
86% |
73% |
54% |
45% |
(95% CI) |
(82, 89) |
(68, 77) |
(48, 60) |
(37, 51) |
Across both studies, 243 of 676 rituximab-treated patients (36%) were 65 years of age or older and 100 rituximab-treated patients (15%) were 70 years of age or older. The results of exploratory subset analyses in elderly patients are presented in Table 12.
| ||||
Age subgroup |
CLL Study 1 |
CLL Study 2 |
||
|
Number of Patients |
Hazard Ratio for PFS (95% CI) |
Number of Patients |
Hazard Ratio for PFS (95% CI) |
|
Age less than 65 yrs |
572 |
0.52 (0.39, 0.70) |
313 |
0.61 (0.45, 0.84) |
Age greater than or |
245 |
0.62 (0.39, 0.99) |
233 |
0.99 (0.70, 1.40) |
Age less than 70 yrs |
736 |
0.51 (0.39, 0.67) |
438 |
0.67 (0.51, 0.87) |
Age greater than or |
81 |
1.17 (0.51, 2.66) |
108 |
1.22 (0.73, 2.04) |
14.6 Rheumatoid Arthritis (RA)
Reducing the Signs and Symptoms: Initial and Retreatment Courses
The efficacy and safety of rituximab were evaluated in two randomized, double-blind, placebo-controlled studies of adult patients with moderately-to-severely-active RA who had a prior inadequate response to at least one TNF inhibitor. Patients were 18 years of age or older, diagnosed with active RA according to American College of Rheumatology (ACR) criteria, and had at least 8 swollen and 8 tender joints.
In RA Study 1 (NCT00468546), patients were randomized to receive either rituximab 2 × 1,000 mg + MTX or placebo + MTX for 24 weeks. Further courses of rituximab 2 × 1,000 mg + MTX were administered in an open‑label extension study at a frequency determined by clinical evaluation, but no sooner than 16 weeks after the preceding course of rituximab. In addition to the intravenous premedication, glucocorticoids were administered orally on a tapering schedule from baseline through Day 14. The proportions of patients achieving ACR 20, 50, and 70 responses at Week 24 of the placebo-controlled period are shown in Table 13.
In RA Study 2 (NCT00266227), all patients received the first course of rituximab 2 × 1,000 mg + MTX. Patients who experienced ongoing disease activity were randomized to receive a second course of either rituximab 2 × 1,000 mg + MTX or placebo + MTX, the majority between Weeks 24–28. The proportions of patients achieving ACR 20, 50, and 70 responses at Week 24, before the retreatment course, and at Week 48, after retreatment, are shown in Table 13.
| ||||||||
Inadequate Response to TNF Antagonists | ||||||||
RA Study 1 |
RA Study 2 |
|||||||
Response |
Placebo |
Rituximab |
Treatment |
Response |
Placebo + |
Rituximab + MTX Retreatment |
||
ACR20 |
ACR20 |
|||||||
Week 24 |
18% |
51% |
33% |
Week 24 |
48% |
45% |
NA |
|
Week 48 |
45% |
54% |
11% |
|||||
ACR50 |
ACR50 |
|||||||
Week 24 |
5% |
27% |
21% |
Week 24 |
27% |
21% |
NA |
|
Week 48 |
26% |
29% |
4% |
|||||
ACR70 |
ACR70 |
|||||||
Week 24 |
1% |
12% |
11% |
Week 24 |
11% |
8% |
NA |
|
Week 48 |
13% |
14% |
1% |
|||||
Improvement was also noted for all components of ACR response following treatment with rituximab, as shown in Table 14.
Inadequate Response to TNF Antagonists | ||||
Placebo + MTX |
Rituximab + MTX |
|||
Parameter |
Baseline |
Wk 24 |
Baseline |
Wk 24 |
Tender Joint Count |
31.0 |
27.0 |
33.0 |
13.0 |
Swollen Joint Count |
20.0 |
19.0 |
21.0 |
9.5 |
Physician Global Assessment* |
71.0 |
69.0 |
71.0 |
36.0 |
Patient Global Assessment* |
73.0 |
68.0 |
71.0 |
41.0 |
Pain* |
68.0 |
68.0 |
67.0 |
38.5 |
Disability Index (HAQ)† |
2.0 |
1.9 |
1.9 |
1.5 |
CRP (mg/dL) |
2.4 |
2.5 |
2.6 |
0.9 |
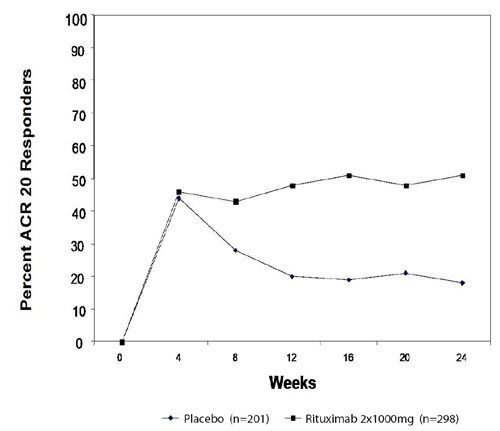
The time course of ACR 20 response for RA Study 1 is shown in Figure 2. Although both treatment groups received a brief course of intravenous and oral glucocorticoids, resulting in similar benefits at Week 4, higher ACR 20 responses were observed for the rituximab group by Week 8. A similar proportion of patients achieved these responses through Week 24 after a single course of treatment (2 infusions) with rituximab. Similar patterns were demonstrated for ACR 50 and 70 responses.
|
Figure 2 |
|
Radiographic Response
In RA Study 1, structural joint damage was assessed radiographically and expressed as changes in Genant-modified Total Sharp Score (TSS) and its components, the erosion score (ES) and the joint space narrowing (JSN) score. Rituximab + MTX slowed the progression of structural damage compared to placebo + MTX after 1 year as shown in Table 15.
Inadequate Response to TNF Antagonists | ||||
Parameter |
Rituximab 2 × 1,000 mg + MTX* |
Placebo + MTX† |
Treatment Difference |
95% CI |
Change during First Year |
||||
TSS |
0.66 |
1.77 |
1.11 |
(0.47, 1.75) |
ES |
0.44 |
1.19 |
0.75 |
(0.32, 1.19) |
JSN Score |
0.22 |
0.58 |
0.36 |
(0.10, 0.62) |
Change during Second Year‡ |
||||
TSS |
0.48 |
1.04 |
— |
— |
ES |
0.28 |
0.62 |
— |
— |
JSN Score |
0.20 |
0.42 |
— |
— |
In RA Study 1 and its open-label extension, 70% of patients initially randomized to rituximab + MTX and 72% of patients initially randomized to placebo + MTX were evaluated radiographically at Year 2. As shown in Table 15, progression of structural damage in rituximab + MTX patients was further reduced in the second year of treatment.
Following 2 years of treatment with rituximab + MTX, 57% of patients had no progression of structural damage. During the first year, 60% of rituximab + MTX treated patients had no progression, defined as a change in TSS of zero or less compared to baseline, compared to 46% of placebo + MTX treated patients. In their second year of treatment with rituximab + MTX, more patients had no progression than in the first year (68% vs. 60%), and 87% of the rituximab + MTX treated patients who had no progression in the first year also had no progression in the second year.
Lesser Efficacy of 500 vs. 1,000 mg Treatment Courses for Radiographic Outcomes
RA Study 3 (NCT00299104) is a randomized, double-blind, placebo-controlled study which evaluated the effect of placebo + MTX compared to rituximab 2 × 500 mg + MTX and rituximab 2 × 1,000 mg + MTX treatment courses in MTX-naïve RA patients with moderately-to-severely-active disease. Patients received a first course of two infusions of rituximab or placebo on Days 1 and 15. MTX was initiated at 7.5 mg/week and escalated up to 20 mg/week by Week 8 in all three treatment arms. After a minimum of 24 weeks, patients with ongoing disease activity were eligible to receive retreatment with additional courses of their assigned treatment. After one year of treatment, the proportion of patients achieving ACR 20/50/70 responses were similar in both rituximab dose groups and were higher than in the placebo group. However, with respect to radiographic scores, only the rituximab 1,000 mg treatment group demonstrated a statistically significant reduction in TSS: a change of 0.36 units compared to 1.08 units for the placebo group, a 67% reduction.
Physical Function Response
RA Study 4 (NCT00299130) is a randomized, double-blind, placebo-controlled study in adult RA patients with moderately-to-severely-active disease with inadequate response to MTX. Patients were randomized to receive an initial course of rituximab 500 mg, rituximab 1,000 mg, or placebo in addition to background MTX.
Physical function was assessed at Weeks 24 and 48 using the Health Assessment Questionnaire Disability Index (HAQ-DI). From baseline to Week 24, a greater proportion of rituximab-treated patients had an improvement in HAQ-DI of at least 0.22 (a minimal clinically important difference) and a greater mean HAQ-DI improvement compared to placebo, as shown in Table 16. HAQ-DI results for the rituximab 500 mg treatment group were similar to the rituximab 1,000 mg treatment group; however radiographic responses were not assessed (see Dosing Precaution in the Radiographic Responses section above). These improvements were maintained at 48 weeks.
Placebo + MTX |
Rituximab |
Treatment Difference |
|
Mean Improvement from Baseline |
0.19 |
0.42 |
0.23 (0.11, 0.34) |
Percent of patients with "Improved" score |
48% |
58% |
11% (0%, 21%) |
14.7 Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
Induction Treatment of Adult Patients with Active Disease (GPA/MPA Study 1)
A total of 197 patients with active, severe GPA and MPA (two forms of ANCA‑Associated Vasculitides) were treated in a randomized, double-blind, active-controlled multicenter, non-inferiority study, conducted in two phases – a 6-month remission induction phase and a 12-month remission maintenance phase. Patients were 15 years of age or older, diagnosed with GPA (75% of patients) or MPA (24% of patients) according to the Chapel Hill Consensus conference criteria (1% of the patients had unknown vasculitis type). All patients had active disease, with a Birmingham Vasculitis Activity Score for Granulomatosis with Polyangiitis (BVAS/GPA) greater than or equal to 3, and their disease was severe, with at least one major item on the BVAS/GPA. Ninety-six (49%) of patients had new disease and 101 (51%) of patients had relapsing disease.
Patients in both arms received 1,000 mg of pulse intravenous methylprednisolone per day for 1 to 3 days within 14 days prior to initial infusion. Patients were randomized in a 1:1 ratio to receive either rituximab 375 mg/m2 once weekly for 4 weeks or oral cyclophosphamide 2 mg/kg daily for 3 to 6 months in the remission induction phase. Patients were pre-medicated with antihistamine and acetaminophen prior to rituximab infusion. Following intravenous corticosteroid administration, all patients received oral prednisone (1 mg/kg/day, not exceeding 80 mg/day) with pre-specified tapering. Once remission was achieved or at the end of the 6-month remission induction period, the cyclophosphamide group received azathioprine to maintain remission. The rituximab group did not receive additional therapy to maintain remission. The main outcome measure for both GPA and MPA patients was achievement of complete remission at 6 months defined as a BVAS/GPA of 0, and off glucocorticoid therapy. The pre-specified non-inferiority margin was a treatment difference of 20%. As shown in Table 17, the study demonstrated non-inferiority of rituximab to cyclophosphamide for complete remission at 6 months.
Rituximab |
Cyclophosphamide |
Treatment Difference |
|
Rate |
64% |
53% |
11% |
95.1%* CI |
(54%, 73%) |
(43%, 63%) |
(-3%, 24%)† |
Complete Remission (CR) at 12 and 18 Months
In the rituximab group, 44% of patients achieved CR at 6 and 12 months, and 38% of patients achieved CR at 6, 12, and 18 months. In patients treated with cyclophosphamide (followed by azathioprine for maintenance of CR), 38% of patients achieved CR at 6 and 12 months, and 31% of patients achieved CR at 6, 12, and 18 months.
Retreatment of Flares with Rituximab
Based upon investigator judgment, 15 patients received a second course of rituximab therapy for treatment of relapse of disease activity which occurred between 8 and 17 months after the induction treatment course of rituximab.
Follow up Treatment of Adult Patients with GPA/MPA who have achieved disease control with other Immunosuppressant (GPA/MPA Study 2)
A total of 115 patients (86 with GPA, 24 with MPA, and 5 with renal-limited ANCA-associated vasculitis) in disease remission were randomized to receive azathioprine (58 patients) or non-U.S.-licensed rituximab (57 patients) in this open-label, prospective, multicenter, randomized, active-controlled study. Eligible patients were 21 years and older and had either newly diagnosed (80%) or relapsing disease (20%). A majority of the patients were ANCA-positive. Remission of active disease was achieved using a combination of glucocorticoids and cyclophosphamide. Within a maximum of 1 month after the last cyclophosphamide dose, eligible patients (based on BVAS of 0), were randomized in a 1:1 ratio to receive either non-U.S.-licensed rituximab or azathioprine.
The non-U.S.-licensed rituximab was administered as two 500 mg intravenous infusions separated by two weeks (on Day 1 and Day 15) followed by a 500 mg intravenous infusion every 6 months for 18 months. Azathioprine was administered orally at a dose of 2 mg/kg/day for 12 months, then 1.5 mg/kg/day for 6 months, and finally 1 mg/kg/day for 4 months; treatment was discontinued after 22 months. Prednisone treatment was tapered and then kept at a low dose (approximately 5 mg per day) for at least 18 months after randomization. Prednisone dose tapering and the decision to stop prednisone treatment after Month 18 were left at the investigator's discretion.
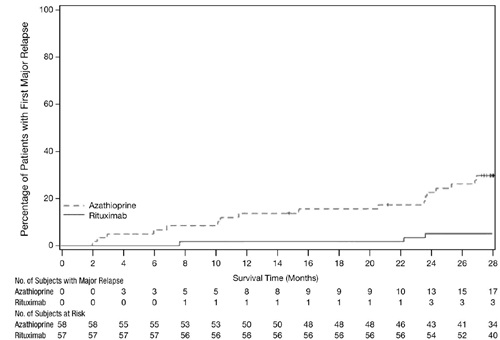
Planned follow-up was until Month 28 (10 or 6 months, respectively, after the last non-U.S.-licensed rituximab infusion or azathioprine dose). The primary endpoint was the occurrence of major relapse (defined by the reappearance of clinical and/or laboratory signs of vasculitis activity that could lead to organ failure or damage, or could be life-threatening) through Month 28.
By Month 28, major relapse occurred in 3 patients (5%) in the non-U.S.-licensed rituximab group and 17 patients (29%) in the azathioprine group.
The observed cumulative incidence rate of first major relapse during the 28 months was lower in patients on non-U.S.-licensed rituximab relative to azathioprine (Figure 3).
| Patients were censored at the last follow up dates if they had no event. |
Figure 3 |
|
14.8 Pemphigus Vulgaris (PV)
PV Study 1 (NCT00784589)
Non-U.S.-licensed rituximab in combination with short-term prednisone was compared to prednisone monotherapy as first-line treatment in 90 newly diagnosed adult patients with moderate to severe pemphigus (74 Pemphigus Vulgaris [PV] and 16 Pemphigus Foliaceus [PF]) in this randomized, open-label, controlled, multicenter study (PV Study 1). Patients were between 19 and 79 years of age and had not received prior therapies for pemphigus. In the PV population, 5 (13%) patients in the group treated with non-U.S.-licensed rituximab and 3 (8%) patients in the prednisone group had moderate disease and 33 (87%) patients in the group treated with non-U.S.-licensed rituximab and 33 (92%) patients in the prednisone group had severe disease according to disease severity defined by Harman’s criteria.
Patients were stratified by baseline disease severity (moderate or severe) and randomized 1:1 to receive either the non-U.S.-licensed rituximab and short-term prednisone or long-term prednisone monotherapy. Patients were pre-medicated with antihistamine, acetaminophen and methylprednisolone prior to infusion of the non-U.S.-licensed rituximab. Patients randomized to the group treated with non-U.S.-licensed rituximab received an initial intravenous infusion of 1,000 mg non-U.S.-licensed rituximab on Study Day 1 in combination with a short-term regimen of 0.5 mg/kg/day oral prednisone tapered off over 3 months if they had moderate disease or 1 mg/kg/day oral prednisone tapered off over 6 months if they had severe disease. All patients received a second intravenous infusion of 1,000 mg non-U.S.-licensed rituximab on Study Day 15. Maintenance infusions of 500 mg non-U.S.-licensed rituximab were administered at Months 12 and 18. Patients randomized to the prednisone monotherapy group received an initial 1 mg/kg/day oral prednisone tapered off over 12 months if they had moderate disease or 1.5 mg/kg/day oral prednisone tapered off over 18 months if they had severe disease. Patients in the group treated with non-U.S.-licensed rituximab who relapsed could receive an additional infusion of 1,000 mg non-U.S.-licensed rituximab in combination with reintroduced or escalated prednisone dose. Maintenance and relapse infusions were administered no sooner than 16 weeks following the previous infusion.
The primary endpoint for the study was complete remission (complete epithelialization and absence of new and/or established lesions) at Month 24 without the use of prednisone therapy for 2 months or more (CRoff for greater than or equal to 2 months).
The results of the trial are presented in Table 18.
|
Non-U.S.-Licensed Rituximab + Short-term Prednisone N=46 |
Prednisone N=44 |
|
|
Number of responders (response rate [%]) |
41 (89%) |
15 (34%) |
PV patients |
34/38 (90%) |
10/36 (28%) |
PF patients |
7/8 (88%) |
5/8 (63%) |
PV Study 2 (NCT02383589)
In a randomized, double-blind, double-dummy, active-comparator, multicenter study, the efficacy and safety of rituximab compared to mycophenolate mofetil (MMF) were evaluated in patients with moderate to severe PV receiving 60-120 mg/day oral prednisone or equivalent (1.0-1.5 mg/kg/day) at study entry and tapered to reach a dose of 60 or 80 mg/day by Day 1. Patients had a confirmed diagnosis of PV within the previous 24 months and evidence of moderate to severe disease defined as a total Pemphigus Disease Area Index (PDAI) activity score of greater than or equal to 15. The study consisted of a screening period of up to 28 days, a 52-week double-blind treatment period, and a 48-week safety follow up period.
One hundred and thirty-five patients were randomized to treatment with rituximab 1,000 mg administered on Day 1, Day 15, Week 24 and Week 26 or oral MMF 2 g/day (starting at 1 g/day on Day 1 and titrated to achieve a goal of 2 g/day by Week 2) for 52 weeks in combination with an initial dose of 60 or 80 mg oral prednisone with the aim of tapering to 0 mg/day by Week 24. Randomization was stratified by duration of PV (within the 1 year prior to screening or greater than 1 year) and geographical region. A dual-assessor approach was used during the study for efficacy and safety evaluations to prevent potential unblinding.
One hundred and twenty-five patients (excluding exploratory data from ten telemedicine patients) were analyzed for efficacy (Modified Intent-to-Treat Population). The primary efficacy endpoint for this study was the proportion of subjects achieving sustained complete remission defined as achieving healing of lesions with no new active lesions (i.e., PDAI activity score of 0) while on 0 mg/day prednisone or equivalent, and maintaining this response for at least 16 consecutive weeks, during the 52-week treatment period.
Secondary endpoints included cumulative oral corticosteroid dose and the total number of disease flares.
The results of the trial are presented in Table 19.
| MMF = Mycophenolate mofetil. CI = Confidence Interval. | |||
|
Rituximab (N=62) |
MMF (N=63) |
Difference (95% CI) |
|
Number of responders (response rate [%]) |
25 (40.3%) |
6 (9.5%) |
30.80% (14.70%, 45.15%) |
Glucocorticoid Exposure
The median (min, max) cumulative oral prednisone dose at Week 52 was 2,775 mg (450; 22,180) in the rituximab group compared to 4,005 mg (900; 19,920) in the MMF group. Topical corticosteroid use and pre-infusion IV methylprednisolone were not included in this analysis. Prior to each infusion, the rituximab group received IV methylprednisolone 100 mg and the MMF group received IV saline solution.
Disease Flare
Disease flare was defined as an appearance of 3 or more new lesions a month that do not heal spontaneously within 1 week or by the extension of established lesions in a patient who has achieved disease control. The total number of disease flares was lower in patients treated with rituximab compared to MMF (6 vs. 44).
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.