(arginine HCl)
Health Professional Information
Dosage and Administration
DOSAGE AND ADMINISTRATION
Adult Dosage
The recommended adult dose is 30 g arginine hydrochloride (300 mL of R-Gene® 10) administered by intravenous infusion over 30 minutes. The total dose should not exceed 30 g arginine hydrochloride. See Directions for Use for preparation instructions.
Pediatric Dosage
The recommended pediatric dose is 0.5 g/kg arginine hydrochloride (5 mL/kg of R-Gene® 10) administered by intravenous infusion over 30 minutes. The total dose should not exceed 30 g arginine hydrochloride.
- •
- For patients weighing 59 kg or less, withdraw a weight based dose from a sealed R-Gene® 10 bottle and place in a separate container for intravenous infusion to avoid the inadvertent delivery and administration of the total volume from the commercially available container. See Directions for Use for preparation instructions.
- •
- For patients weighing 60 kg or more, the recommended dose is 30 g arginine hydrochloride (300 mL of R-Gene® 10). See Directions for Use for preparation instructions
Test Procedure
The intravenous infusion of R-Gene® 10 is a part of the test for measurement of pituitary reserve of human growth hormone and, for successful administration of the test, clinical conditions and procedures should be as follows:
- 1.
- The test should be scheduled in the morning following a normal night's sleep, and an overnight fast should continue through the test period.
- 2.
- Patients must be placed at bed rest for at least 30 minutes before the infusion begins. Care should be taken to minimize apprehension and distress. This is particularly important in children.
- 3.
- R-Gene® 10 (Arginine Hydrochloride Injection, USP) is a hypertonic solution and should only be infused through an indwelling needle or soft catheter placed in an antecubital vein or other suitable vein (see PRECAUTIONS). Blood samples should be taken by venipuncture from the contra-lateral arm.
- 4.
- A desirable schedule for drawing blood samples is at −30, 0, 30, 60, 90, 120 and 150 minutes.
- 5.
- R-Gene® 10 should be infused beginning at zero time at a uniform rate which will permit the recommended dose to be administered over 30 minutes.
- 6.
- Blood samples should be promptly centrifuged and the plasma stored at −20°C until assayed by one of the published radioimmunoassay procedures.
- 7.
- Diagnostic test results showing a deficiency of pituitary reserve for HGH should be confirmed by a second test with R-Gene® 10, or one may elect to confirm with the insulin hypoglycemia test. A waiting period of one day is advised between tests.
Directions for Use
R-Gene® 10 is provided as a ready-to-use solution for patients weighing 60 kg (132 lbs) or more and should not be further diluted. For pediatric patients weighing 59 kg (130 lbs) or less a dose must be placed in a separate container. Follow the preparation instructions below.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
For Pediatric Patients weighing 59 kg (130 lbs) or less:
Withdraw a weight-based dose from an intact sealed bottle of R-Gene® 10. The entire 300 mL bottle of R-Gene® 10 for infusion is not intended for use in patients weighing 59 kg or less. The dose must be placed in a separate container, such as an evacuated sterile glass container designed for intravenous administration, using aseptic technique.
Additionally, R-Gene® 10 is stable in polypropylene syringes and plastic containers made of either polyvinyl chloride (PVC) or ethylene vinyl acetate (EVA).
The post-penetration storage period is not more than 4 hours including infusion time at room temperature or 24 hours at refrigerated temperature (2-8°C).
The healthcare professional administering the dose should verify the accuracy of the dose prior to administration.
Use only if the solution is clear. Discard any unused drug product.
For Adults and Pediatric Patients weighing 60kg (132 lbs) or more:
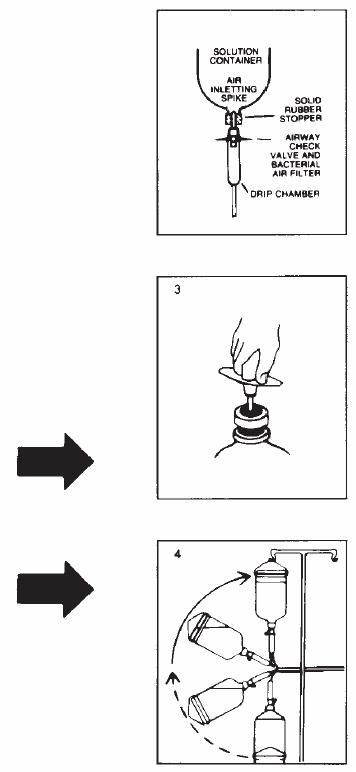
Follow these directions using aseptic technique. As R-Gene® 10 for intravenous use is provided in glass containers, a standard air-inletting, air-filtering intravenous infusion set with a bacterial air filter is required.
- 1.
- Use only if solution is clear and seal is intact. Carefully examine bottle for evidence of damage, e.g., small cracks, dents in seal, or areas of dried powder on exterior. Do not administer contents if such damage is found.
- 2.
- Remove plastic flip off lid from bottle to expose rubber stopper, taking care that you do not contaminate the target site of the stopper with fingers, hair, clothing, etc. Immediately perform step #3.
- 3.
- With shut-off clamp closed, remove sterility protector from spike of administration set and immediately insert set with a quick thrust into center of stopper with bottle upright on table. (Push straight in — don't twist — twisting may cause stopper coring.)
- 4.
- Promptly invert bottle to automatically establish fluid level in drip chamber and to check for vacuum by observing rising filtered air bubbles. Discard bottle if there is no vacuum or if the solution is not clear.
- 5.
- Clear tubing of air. Proceed with infusion.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.