(nifedipine extended release tablets)
Health Professional Information
Description
DESCRIPTION
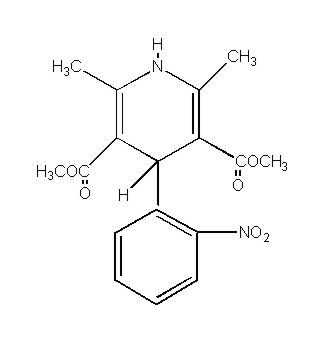
Nifedipine is a drug belonging to a class of pharmacological agents known as the calcium channel blockers. Nifedipine is 3,5-pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-, dimethyl ester, C17H18N2O6, and has the structural formula:
Nifedipine is a yellow crystalline substance, practically insoluble in water but soluble in ethanol. It has a molecular weight of 346.3. PROCARDIA XL is a registered trademark for Nifedipine GITS. Nifedipine GITS (Gastrointestinal Therapeutic System) Tablet is formulated as a once-a-day controlled-release tablet for oral administration to provide 30, 60, or 90 mg of nifedipine.
Each tablet contains 33 mg nifedipine to provide a 30 mg dose.
Each tablet contains 66 mg nifedipine to provide a 60 mg dose.
Each tablet contains 99 mg nifedipine to provide a 90 mg dose.
Inert ingredients in the formulations are: cellulose acetate; hydroxypropyl cellulose; hypromellose; magnesium stearate; polyethylene glycol; polyethylene oxide; red ferric oxide; sodium chloride; titanium dioxide.
System Components and Performance
PROCARDIA XL® Extended Release Tablet is similar in appearance to a conventional tablet. It consists, however, of a semipermeable membrane surrounding an osmotically active drug core. The core itself is divided into two layers: an "active" layer containing the drug, and a "push" layer containing pharmacologically inert (but osmotically active) components. As water from the gastrointestinal tract enters the tablet, pressure increases in the osmotic layer and "pushes" against the drug layer, releasing drug through the precision laser-drilled tablet orifice in the active layer.
PROCARDIA XL Extended Release Tablet is designed to provide nifedipine at an approximately constant rate over 24 hours. This controlled rate of drug delivery into the gastrointestinal lumen is independent of pH or gastrointestinal motility. PROCARDIA XL depends for its action on the existence of an osmotic gradient between the contents of the bi-layer core and fluid in the gastrointestinal tract. Drug delivery is essentially constant as long as the osmotic gradient remains constant, and then gradually falls to zero. Upon swallowing, the biologically inert components of the tablet remain intact during gastrointestinal transit and are eliminated in the feces as an insoluble shell.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.