(pneumococcal 20-valent conjugate vaccine)
Health Professional Information
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
For intramuscular administration only.
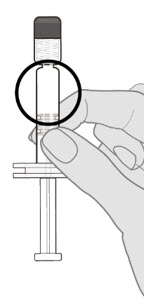
2.1 Preparation
Do not mix Prevnar 20 with other vaccines/products in the same syringe.
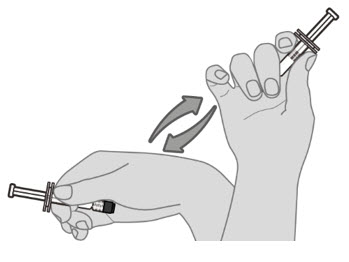
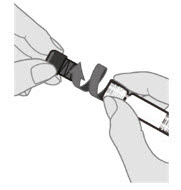
2.2 Administration
For intramuscular injection only.
Each 0.5-mL dose is to be injected intramuscularly using a sterile needle attached to the supplied pre-filled syringe.
2.3 Vaccination Schedule for Individuals 6 Weeks Through 15 Months of Age
Administer Prevnar 20 as a 4-dose series at 2, 4, 6, and 12 through 15 months of age (and at least 2 months after the third dose). The first dose may be given as early as 6 weeks of age.
2.4 Catch-Up Vaccination Schedule for Unvaccinated Individuals 7 Months Through 17 Years of Age
Individuals 7 months through 17 years of age who have never received a pneumococcal conjugate vaccine may receive Prevnar 20 according to the schedule in Table 1:
| |
Age at First Dose |
Total Number of 0.5-mL Doses |
7 through 11 months of age |
3† |
12 through 23 months of age |
2‡ |
24 months of age and above |
1 |
2.5 Catch-Up Vaccination Schedule for Individuals Previously Vaccinated With One or More Doses of a Lower Valency Pneumococcal Conjugate Vaccine
Administer a single dose of Prevnar 20 to individuals 15 months through 17 years of age previously vaccinated with one or more doses of a lower valency pneumococcal conjugate vaccine. The dose of Prevnar 20 should be administered at least 8 weeks after the last dose of the lower valency pneumococcal conjugate vaccine.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.