Health Professional Information
Health Professional Information
Instructions for Use
INSTRUCTIONS FOR USE
XALKORI® [zal-KOR-ee]
(crizotinib)
oral pellets
This Instructions for Use contains information on how to give or take XALKORI oral pellets. Read this Instructions for Use each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider or pharmacist about your or your child’s medical condition or treatment.
Important information you need to know before giving or taking XALKORI oral pellets:
- •
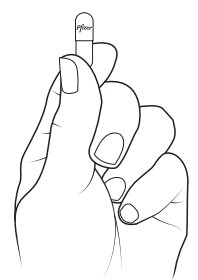
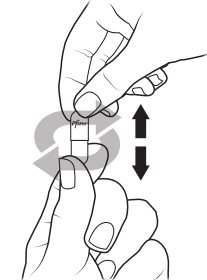
- XALKORI oral pellets come in a capsule “shell” that must be opened before giving or taking a dose. Do not swallow the shell containing the oral pellets. Do not chew or crush the oral pellets.
- •
- XALKORI oral pellets come in 3 dosage strengths: 20 mg, 50 mg, and 150 mg. Your healthcare provider may combine different strengths for your prescribed dose. No more than 4 XALKORI oral pellet shells are to be used for a single dose.
- •
- Your healthcare provider will decide the right dose of XALKORI oral pellets for you or your child. Follow your healthcare provider’s instructions for the dose of XALKORI oral pellets to give your child or for you to take.
- •
- Empty XALKORI oral pellets from the shells as described in Steps 1 to 4 below.
- •
- Check the expiration date on the bottle containing XALKORI oral pellets. Do not use XALKORI oral pellets after the expiration date on the bottle has passed.
- •
- Ask your healthcare provider or pharmacist if you are not sure how to prepare and give or take the prescribed dose of XALKORI oral pellets.
Supplies needed to give or take XALKORI oral pellets:
- •
- XALKORI oral pellet(s), as prescribed by your healthcare provider.
- •
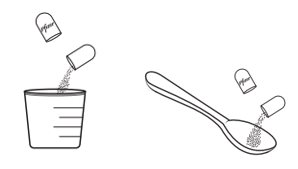
- Spoon or medicine cup (optional). See Step 4 “Giving or taking XALKORI oral pellets”.
Preparing XALKORI oral pellets (Steps 1 to 3):
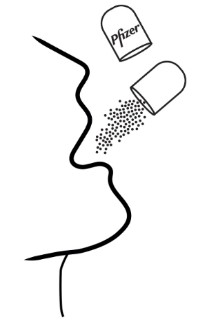
Giving or taking XALKORI oral pellets (Step 4): There are 2 options for giving or taking the oral pellets.
Storing XALKORI oral pellets:
- •
- Store XALKORI oral pellets at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Keep XALKORI oral pellets and all medicines out of the reach of children.
Disposing of empty XALKORI oral pellet shells:
- •
- Dispose of (throw away) the empty XALKORI oral pellet shell(s) in the household trash.
- •
- Ask your pharmacist how to throw away medicines you no longer use or are expired.
For more information, go to www.Pfizer.com or call 1-800-438-1985.
LAB-1526-1.0
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 09/2023
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.