(talazoparib)
Medication Guide
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Prescribing Information
Health Professional Information
Description
11 DESCRIPTION
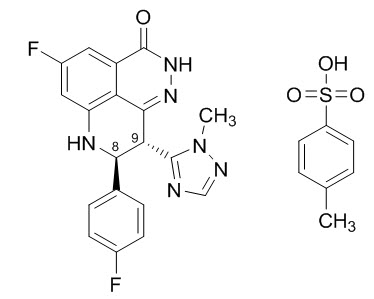
Talazoparib is an inhibitor of mammalian polyadenosine 5'-diphosphoribose (ADP-ribose) polymerase (PARP) enzymes. TALZENNA contains talazoparib tosylate. The chemical name of talazoparib tosylate is (8S,9R)-5-Fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-2,7,8,9-tetrahydro-3H-pyrido[4,3,2-de]phthalazin-3-one 4-methylbenzenesulfonate (1:1). The chemical formula of talazoparib tosylate is C26H22F2N6O4S, and the relative molecular mass is 552.56 Daltons. The chemical structure of talazoparib tosylate is shown below:
Talazoparib tosylate is a white to yellow solid. TALZENNA capsules for oral use are available as liquid-filled soft gelatin capsules. Each capsule contains 0.1 mg, 0.25 mg, 0.35 mg, 0.5 mg, 0.75, or 1 mg of talazoparib equivalent to 0.145 mg, 0.363 mg, 0.509 mg, 0.727 mg, 1.09 mg, or 1.453 mg talazoparib tosylate, respectively.
TALZENNA capsules contain the following inactive ingredients: gelatin, glycerol, iron oxide (red), iron oxide (yellow), polyethylene glycol 400, purified water, sorbitol, titanium dioxide, and tocopherol.
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.