Health Professional Information
Health Professional Information
How should I use PREMARIN vaginal cream?
PREMARIN vaginal cream is a cream that you place in your vagina with the applicator provided with the cream.
- •
- Take the dose recommended by your healthcare provider and talk to him or her about how well that dose is working for you
- •
- Estrogens should be used at the lowest dose possible for your treatment only as long as needed. You and your healthcare provider should talk regularly (for example, every 3 to 6 months) about the dose you are using and whether you still need treatment with PREMARIN vaginal cream
- •
- Step 1. Remove cap from tube.
- •
- Step 2. Screw nozzle end of applicator onto tube (Figure A).
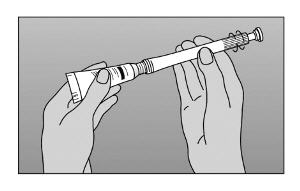
- Step 3. Gently squeeze tube from the bottom to force sufficient cream into the barrel to provide the prescribed dose. Use the marked stopping points on the applicator to measure the correct dose, as prescribed by your healthcare provider (Figure B).
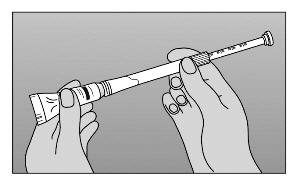
- Step 4. Unscrew applicator from tube.
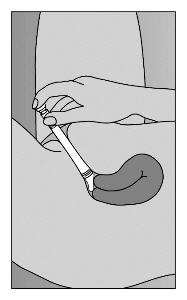
- Step 5. Lie on back with knees drawn up. To deliver medication, gently insert applicator deeply into vagina and press plunger downward to its original position (Figure C).
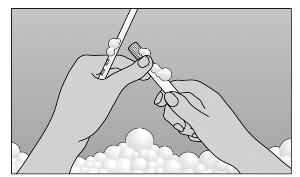
- Step 6. TO CLEANSE: Pull plunger to remove it from barrel. Wash with mild soap and warm water (Figure D).
- DO NOT BOIL OR USE HOT WATER.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.