Health Professional Information
Health Professional Information
How should I take PAXLOVID?
- •
- Take PAXLOVID exactly as your healthcare provider tells you to take it.
- •
-
PAXLOVID consists of 2 medicines: nirmatrelvir tablets and ritonavir tablets. The 2 medicines are taken together for 5 days.
- o
- Nirmatrelvir is an oval, pink tablet.
- o
- Ritonavir is a white or off-white tablet.
- •
- PAXLOVID is available in 3 Dose Packs (see Figures A, B, and C below). Your healthcare provider will prescribe the PAXLOVID Dose Pack that is right for you. Follow the instruction for the Dose Pack you receive.
- •
- If you have kidney disease, your healthcare provider may prescribe a lower dose (see Figures B and C). Talk to your healthcare provider to make sure you receive the correct Dose Pack.
If you are prescribed PAXLOVID 300 mg; 100 mg Dose Pack
Each dose contains 3 tablets taken together twice daily
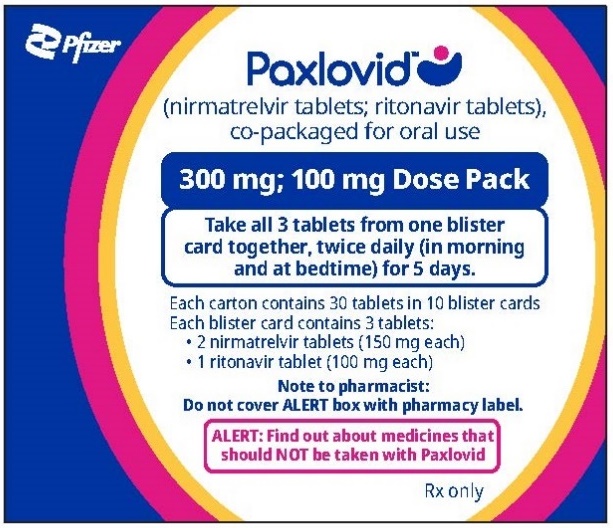
How to take PAXLOVID 300 mg; 100 mg Dose Pack
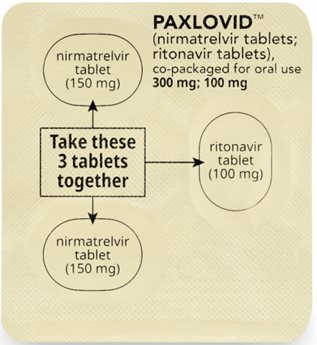
Morning Dose:
Take the 2 pink nirmatrelvir tablets and
1 white to off-white ritonavir tablet together.

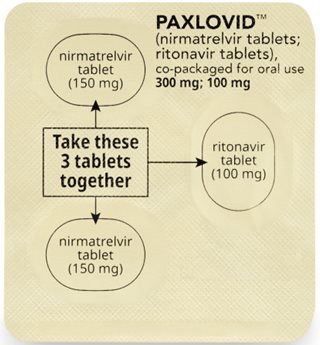
Bedtime Dose:
Take the 2 pink nirmatrelvir tablets and
1 white to off-white ritonavir tablet together.

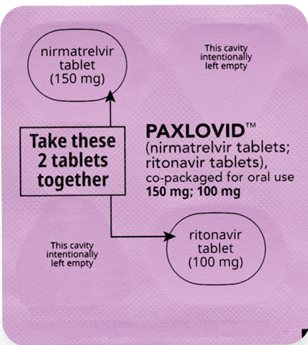
If you are prescribed PAXLOVID 150 mg; 100 mg Dose Pack
Each dose contains 2 tablets taken together twice daily

How to take PAXLOVID 150 mg; 100 mg Dose Pack
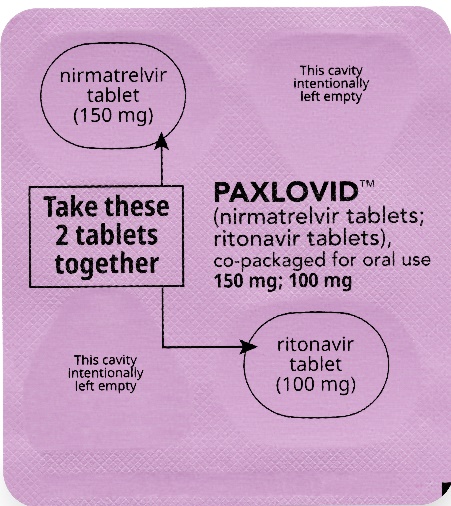
Morning Dose:
Take the 1 pink nirmatrelvir tablet and
1 white ritonavir tablet together.

Bedtime Dose:
Take the 1 pink nirmatrelvir tablet and
1 white ritonavir tablet together.

If you are prescribed PAXLOVID 300 mg; 100 mg (Day 1) and 150 mg; 100 mg (Days 2-5)
Each dose is taken together once daily; on days of dialysis take PAXLOVID after receiving dialysis

How to take PAXLOVID 300 mg; 100 mg (Day 1) and 150 mg; 100 mg (Days 2-5)
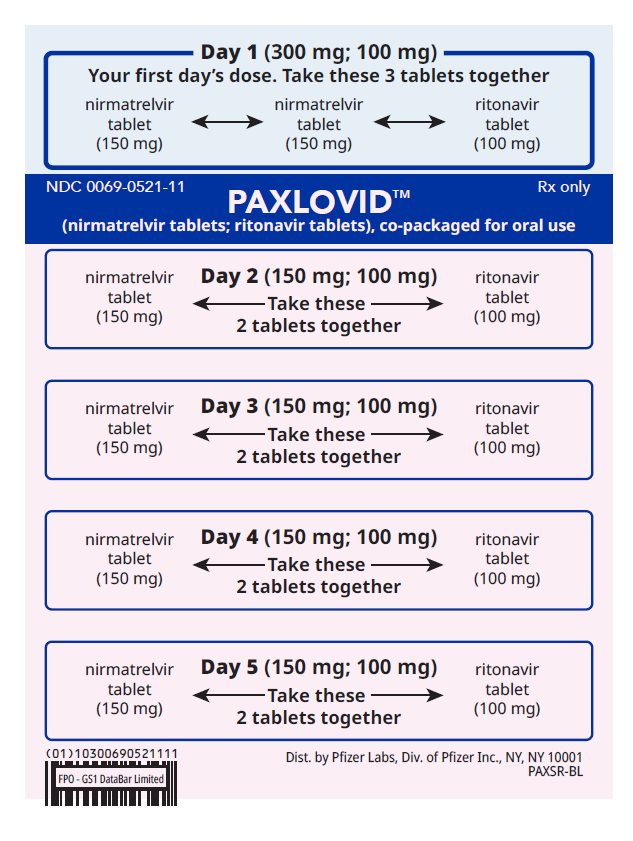
Day 1 (First Day):
Take the 2 pink nirmatrelvir tablets and
1 white ritonavir tablet together
(Blue part of the blister card).
Days 2-5:
Take the 1 pink nirmatrelvir tablet and
1 white ritonavir tablet together
- •
- Do not remove your PAXLOVID tablets from the blister card before you are ready to take your dose.
- •
- If you are taking PAXLOVID tablets twice daily (Figure A or Figure B), take your first dose of PAXLOVID in the morning or at bedtime, depending on when you pick up your prescription, or as your healthcare provider tells you to. Take your doses at around the same time each day.
- •
- If you have severe kidney disease and are taking PAXLOVID tablets once daily (Figure C), follow the daily dose instruction on the blister card. Take your dose at around the same time each day.
- •
- Swallow the tablets whole. Do not chew, break, or crush the tablets.
- •
- Take PAXLOVID with or without food.
- •
- Do not stop taking PAXLOVID without talking to your healthcare provider, even if you feel better.
- •
- If you miss a dose of PAXLOVID within 8 hours of the time it is usually taken, take it as soon as you remember. If you miss a dose by more than 8 hours, skip the missed dose and take the next dose at your regular time. Do not take 2 doses of PAXLOVID at the same time.
- •
- If you take too much PAXLOVID, call your healthcare provider or go to the nearest hospital emergency room right away.
- •
- If you are taking a ritonavir- or cobicistat-containing medicine to treat hepatitis C or HIV-1 infection, you should continue to take your medicine as prescribed by your healthcare provider.
Talk to your healthcare provider if you do not feel better or if you feel worse after 5 days.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.
