Health Professional Information
Health Professional Information
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
- •
- Alprostadil is available in different strengths and presentations. Determine the most suitable dose and presentation for each patient.
- •
- CAVERJECT is available in single-dose vials containing 20 mcg or 40 mcg of alprostadil. Make sure a new, correct strength vial of CAVERJECT is used for each patient dosage preparation.
- •
- Titrate the dose of CAVERJECT for each patient to the lowest effective dose.
- •
- CAVERJECT doses greater than 60 mcg are not recommended.
- •
- Administer the first injections of CAVERJECT in the health care provider’s office by medically trained personnel.
- •
- Instruct the patient on proper use and assess that they are well trained in the self-injection technique prior to initiation of home use. Refer to the Patient Information and Instructions for Use.
- •
- Re-evaluate patients regularly (every 3 months or as clinically appropriate) and determine if dosage adjustments are needed.
2.2 Recommended Dosage for Erectile Dysfunction
Erectile Dysfunction of Vasculogenic, Psychogenic, or Mixed Etiology
- •
- Initiate dosing with 2.5 mcg of CAVERJECT intracavernosally, as recommended [see Dosage and Administration (2.4)].
- •
- If there is a partial response at 2.5 mcg, administer another dose of 2.5 mcg within 1 hour. Use a new vial for each dose of CAVERJECT.
- •
- During titration, no more than 2 doses should be given within a 24-hour period.
- •
- If additional titration is required, administer doses in increments of 5 to 10 mcg at least 24 hours apart.
- •
- The optimal dose should produce an erection suitable for intercourse that does not exceed a duration of 1 hour.
- •
- The patient must stay in the health care provider’s office until complete detumescence occurs.
Repeat the titration as necessary until the optimal dose is achieved. Doses greater than 60 mcg are not recommended.
Erectile Dysfunction of Pure Neurogenic Etiology (e.g., Spinal Cord Injury).
- •
- Initiate dosing with 1.25 mcg of CAVERJECT intracavernosally, as recommended [see Dosage and Administration (2.4)].
- •
- If there is a partial response at 1.25 mcg, administer another dose of 1.25 mcg within 1 hour. Use a new vial for each dose of CAVERJECT.
- •
- If additional titration is required, administer a dose of 5 mcg at least 24 hours later.
- •
- During titration, no more than 2 doses should be given within a 24-hour period.
- •
- The optimal dose should produce an erection suitable for intercourse that does not exceed a duration of 1 hour.
- •
- The patient must stay in the health care provider’s office until complete detumescence occurs.
Repeat the titration as necessary until the optimal dose is achieved. Doses greater than 60 mcg are not recommended.
Maintenance Dosage for Patient Home Use
- •
- Once the dose of CAVERJECT has been determined in the health care provider’s office, additional dose adjustment may be required after consultation with the health care provider. Adjust the dose in accordance with the titration guidelines described above.
- •
- The recommended frequency of injection is no more than 3 times weekly, with at least 24 hours between each dose.
Adjunct to the Diagnosis of Erectile Dysfunction
As an adjunct to the diagnosis of erectile dysfunction, inject CAVERJECT intracavernosally and monitor patients for the occurrence of an erection. Extensions of this testing are the use of CAVERJECT as an adjunct to laboratory investigations, such as duplex or Doppler ultrasound imaging. For any of these tests, use a single dose of CAVERJECT that induces a rigid erection. Use the dose regimen for ‘Erectile Dysfunction of Vasculogenic, Psychogenic, or Mixed Etiology’ above.
2.3 Preparation Instructions
Supplies Needed and Not Supplied With CAVERJECT
- •
- 1 mL of the diluent (bacteriostatic water for injection)
- •
- 1 mL to 3 mL syringe, dependent on the titrated dose
- •
- 21 to 27 gauge needle for reconstitution
- •
- 29 or 30 gauge one-half inch needle for injection
- •
- alcohol swabs
Reconstitution Instructions
- •
- CAVERJECT vial(s): Using the correct strength vial containing 20 mcg or 40 mcg of CAVERJECT, use a 1 mL to 3 mL syringe, a 21 to 27 gauge needle, and 1 mL of the diluent (bacteriostatic water for injection) for reconstitution. Reconstitution results in CAVERJECT 20 mcg/mL or 40 mcg/mL.
- •
- Visually inspect the solution in the vial for particulate matter and discoloration. Do not use the solution if it is cloudy, colored or contains particles.
| 20 mcg Vial | 40 mcg Vial | ||
|---|---|---|---|
| Dose | Volume to Inject | Dose | Volume to Inject |
1.25 mcg | 0.06 mL | 1.25 mcg | --- |
2.5 mcg | 0.125 mL | 2.5 mcg | --- |
5 mcg | 0.25 mL | 5 mcg | 0.125 mL |
10 mcg | 0.5 mL | 10 mcg | 0.25 mL |
15 mcg | 0.75 mL | 15 mcg | 0.375 mL |
20 mcg | 1 mL | 20 mcg | 0.5 mL |
25 mcg | --- | 25 mcg | 0.625 mL |
30 mcg | --- | 30 mcg | 0.75 mL |
40 mcg | --- | 40 mcg | 1 mL |
- •
- Draw the dose of CAVERJECT into the syringe.
- •
- Replace the needle used for reconstitution with a 29 or 30 gauge one-half inch needle prior to injection.
- •
- The reconstituted solution should be used within 24 hours when stored at or below 25°C (77°F).
Refer to the Patient Information and Instructions for Use in the FDA-approved patient labeling for the complete detailed instructions on reconstitution and needle preparation steps.
2.4 Administration Instructions
- •
- Use a 29 or 30 gauge one-half inch needle for injecting each dose.
- •
- The patient should be in a sitting or slightly reclined position when injecting a dose.
- •
- Retract the foreskin in uncircumcised patients.
- •
- Grasp the head of the penis with the thumb and forefinger and stretch it lengthwise along the thigh.
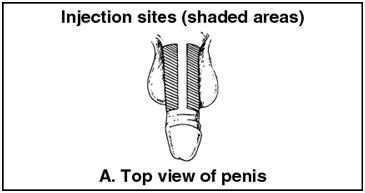
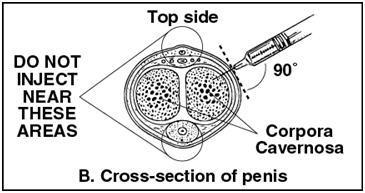
- •
- The site of injection is either the right or left lateral penis. See Figures A and B below.
Figure A | Figure B |
- •
- Wipe the intended injection site with an alcohol swab prior to injection.
- •
- Insert needle perpendicular to the long dorsolateral penile axis in the proximal third of penis. Avoid angulation of the syringe and do not bend the needle.
- •
- Avoid visible veins during injection.
- •
- With each use of CAVERJECT, alternate the side of the penis that is injected.
- •
- Compress the site of injection with an alcohol swab or sterile gauze for 5 minutes.
- •
- CAVERJECT is intended for single patient use only and should be discarded after use.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.