(ritlecitinib)
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
The efficacy and safety of LITFULO were evaluated in one randomized, double-blind, placebo-controlled trial (Trial AA-I) in subjects 12 years of age and older with alopecia areata with ≥50% scalp hair loss, including alopecia totalis (AT) and alopecia universalis (AU).
Trial AA-I evaluated a total of 718 subjects who were randomized to one of the following treatment regimens for 48 weeks: 1) 200 mg once daily for 4 weeks followed by 50 mg once daily for 44 weeks; 2) 200 mg once daily for 4 weeks followed by 30 mg once daily for 44 weeks; 3) 50 mg once daily for 48 weeks; 4) 30 mg once daily for 48 weeks; 5) 10 mg once daily for 48 weeks; 6) placebo for 24 weeks followed by 200 mg once daily for 4 weeks and 50 mg once daily for 20 weeks; or 7) placebo for 24 weeks followed by 50 mg once daily for 24 weeks.
The recommended dose of LITFULO is 50 mg once daily and the results for this dose are discussed below.
Across all treatment groups 62% of subjects were female, 68% were White, 26% were Asian, and 4% were Black or African American. The majority of subjects (85%) were adults (≥18 years of age) with a mean age of 33.7 years. A total of 105 (15%) subjects 12 to <18 years of age and 20 (3%) subjects 65 years of age and older were enrolled. The mean baseline Severity of Alopecia Tool (SALT) score ranged from 88.3 to 93.0 across treatment groups; among subjects without AT/AU at baseline, the mean SALT score ranged from 78.3 to 87.0. The majority of subjects had abnormal eyebrows (83%) and eyelashes (75%) at baseline across treatment groups. The median duration since alopecia areata diagnosis was 6.9 years and the median duration of the current alopecia areata episode was 2.5 years. Randomization was stratified by AT/AU status with 46% of subjects classified as AT/AU based upon a baseline SALT score of 100.
Clinical Response
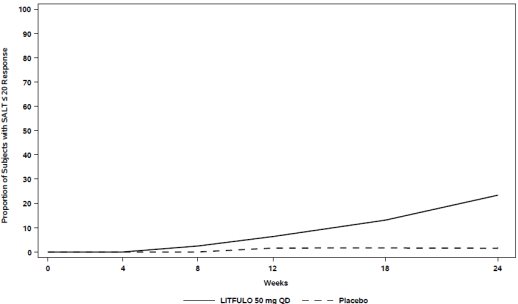
Assessment of scalp hair loss was based on the SALT score. At Week 24, a greater proportion of subjects had a SALT ≤20 response (20% or less of scalp hair loss) and SALT ≤10 response (10% or less of scalp hair loss) with LITFULO compared to placebo (Table 7). The percentage of subjects achieving SALT ≤20 response by visit is shown in Figure 1.
| LITFULO 50 mg QD (N=130) % Responders | Placebo (N=131) % Responders | Difference from Placebo (95% CI) | |
|---|---|---|---|
| Abbreviations: CI = confidence interval; N = total number of subjects; QD = once daily; SALT = Severity of Alopecia Tool. | |||
SALT ≤20 response* | 23.0 | 1.6 | 21.4 (13.4, 29.5) |
SALT ≤10 response† | 13.4 | 1.5 | 11.9 (5.4, 18.3) |
Figure 1. SALT ≤20 Response through Week 24
| Abbreviations: QD = once daily; SALT = Severity of Alopecia Tool. |
 |
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.