Health Professional Information
Description
11 DESCRIPTION
Lidocaine Hydrochloride Injection, USP contains lidocaine hydrochloride, an amide local anesthetic, as the active pharmaceutical ingredient. The route of administration for Lidocaine Hydrochloride Injection is by injection, for infiltration, nerve block, epidural and caudal use.
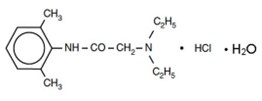
Lidocaine hydrochloride is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, monohydrochloride monohydrate and has the molecular weight of 288.8 g/mol. Lidocaine hydrochloride molecular formula is C14H22N2O • HCl•H2O, and has the following structural formula:
Each single-dose vial contains Lidocaine Hydrochloride Injection, USP, which is a sterile, nonpyrogenic, clear, colorless aqueous solution intended for parenteral administration. [see Indications and Usage (1)] for special uses.
Each mL contains 20 mg lidocaine hydrochloride and 6 mg sodium chloride, in Water for Injection. Sodium hydroxide and/or hydrochloric acid may be used to adjust pH between 5.0 and 7.0.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.