Health Professional Information
Description
11 DESCRIPTION
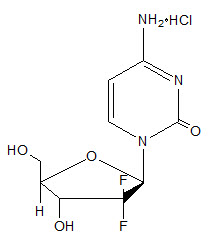
Gemcitabine is a nucleoside metabolic inhibitor. Gemcitabine hydrochloride is 2´-deoxy-2´,2´-difluorocytidine monohydrochloride (β-isomer) with the following structural formula:
The empirical formula for gemcitabine hydrochloride is C9H11F2N3O4 ∙ HCl. It has a molecular weight of 299.66 g/mol.
Gemcitabine hydrochloride is soluble in water, slightly soluble in methanol, and practically insoluble in ethanol and polar organic solvents.
Gemcitabine hydrochloride is a sterile white to off-white lyophilized powder and available as 2 g single-dose vials for intravenous use only. Each 2 g vial contains 2 g of gemcitabine hydrochloride (expressed as free base), 2 g mannitol, and 125 mg sodium acetate. Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.