Health Professional Information
Description
DESCRIPTION
Fluconazole, the first of a new subclass of synthetic triazole antifungal agents, is available as a sterile solution for intravenous use in flexible plastic containers.
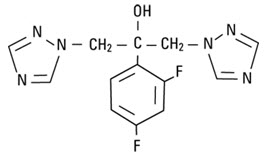
Fluconazole is designated chemically as 2,4-difluoro-α,α1-bis(1H-1,2,4-triazol-1-ylmethyl) benzyl alcohol with an empirical formula of C13H12F2N6O and molecular weight of 306.3. The structural formula is:
Fluconazole is a white crystalline solid which is slightly soluble in water and saline.
Fluconazole in Sodium Chloride Injection, USP is an iso-osmotic, sterile, nonpyrogenic solution. Each mL contains 2 mg of fluconazole and 9 mg of sodium chloride. The pH ranges from 4.0 to 8.0 in the sodium chloride diluent. Injection volumes of 100 mL and 200 mL are packaged in flexible plastic containers.
The flexible plastic container is fabricated from a specially formulated polyvinyl chloride. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexylphthalate (DEHP), up to 5 parts per million. However, the suitability of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.