(etrasimod)
Health Professional Information
Description
11 DESCRIPTION
VELSIPITY contains etrasimod, a sphingosine 1-phosphate (S1P) receptor modulator, supplied as etrasimod arginine.
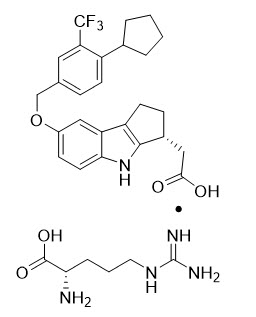
Etrasimod arginine is a white, off-white to light brown solid that is slightly soluble in water. The chemical name of etrasimod arginine is L-Arginine, (3R)-7-[[4-cyclopentyl-3-(trifluoromethyl)phenyl]methoxy]-1,2,3,4-tetrahydrocyclopent[b]indole-3-acetate (1:1) having a molecular formula of C32H40F3N5O5 and a molecular weight of 631.69 g/mol.
The chemical structure of etrasimod arginine is:
VELSIPITY is supplied for oral administration as 2 mg tablets. Each tablet contains 2 mg etrasimod (equivalent to 2.76 mg etrasimod arginine) and the following inactive ingredients: magnesium stearate, mannitol, microcrystalline cellulose, and sodium starch glycolate, with a film coating containing FD&C blue #1/brilliant blue FCF aluminum lake, FD&C blue #2/indigo carmine aluminum lake, FD&C yellow #5/tartrazine aluminum lake, macrogol 4000 JP/PEG 3350, polyvinyl alcohol (partially hydrolyzed), talc, and titanium dioxide.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.