(doxorubicin hydrochloride)
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
14.1 Adjuvant Breast Cancer
The efficacy of doxorubicin hydrochloride-containing regimens for the post-operative, adjuvant treatment of surgically resected breast cancer was evaluated in a meta-analysis conducted by the Early Breast Cancer Trialists Collaborative Group (EBCTCG). The EBCTCG meta-analyses compared cyclophosphamide, methotrexate, and fluorouracil (CMF) to no chemotherapy (19 trials including 7523 patients) and doxorubicin hydrochloride-containing regimens with CMF as an active control (6 trials including 3510 patients). Data from the meta-analysis of trials comparing CMF to no therapy were used to establish the historical treatment effect size for CMF regimens. The major efficacy outcome measures were disease-free survival (DFS) and overall survival (OS).
Of the 3510 women (2157 received doxorubicin hydrochloride-containing regimens and 1353 received CMF treatment) with early breast cancer involving axillary lymph nodes included in the six trials from the meta-analyses, approximately 70% were premenopausal and 30% were postmenopausal.
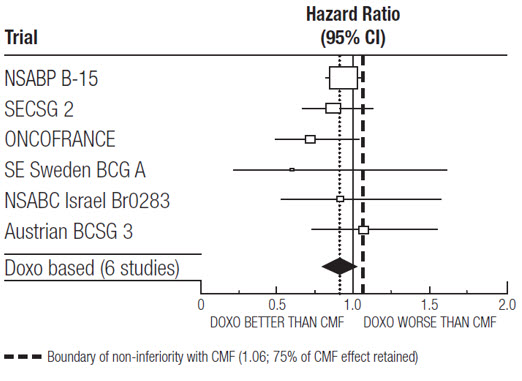
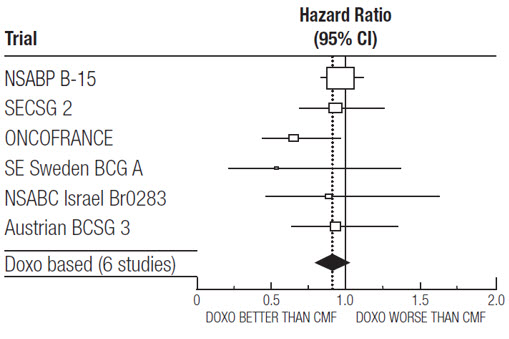
At the time of the meta-analysis, 1745 first recurrences and 1348 deaths had occurred. The analyses demonstrated that doxorubicin hydrochloride-containing regimens retained at least 75% of the historical CMF adjuvant effect on DFS with a hazard ratio (HR) of 0.91 (95% CI: 0.82, 1.01) and on OS with a HR of 0.91 (95% CI: 0.81, 1.03). Efficacy results are provided in Table 3 and Figures 1 and 2.
| Study (starting year) | Regimens | No. of Cycles | No. of Patients | Doxorubicin Hydrochloride-Containing Regimens vs. CMF HR* (95% CI) | |
|---|---|---|---|---|---|
| DFS | OS | ||||
| Abbreviations: DFS = disease free survival; OS = overall survival; AC = doxorubicin hydrochloride, cyclophosphamide; AVbCMF = doxorubicin hydrochloride, vinblastine, cyclophosphamide, methotrexate, fluorouracil; CMF = cyclophosphamide, methotrexate, fluorouracil; CMFVA = cyclophosphamide, methotrexate, fluorouracil, vincristine, doxorubicin hydrochloride; FAC = fluorouracil, doxorubicin hydrochloride, cyclophosphamide; FACV = fluorouracil, doxorubicin hydrochloride, cyclophosphamide, vincristine; HR = hazard ratio; CI = confidence interval | |||||
| |||||
NSABP B-15 | AC | 4 | 1562† | 0.93 (0.82, 1.06) | 0.97 (0.83, 1.12) |
CMF | 6 | 776 | |||
SECSG 2 | FAC | 6 | 260 | 0.86 (0.66, 1.13) | 0.93 (0.69, 1.26) |
CMF | 6 | 268 | |||
ONCOFRANCE | FACV | 12 | 138 | 0.71 (0.49, 1.03) | 0.65 (0.44, 0.96) |
CMF | 12 | 113 | |||
SE Sweden BCG A | AC | 6 | 21 | 0.59 (0.22, 1.61) | 0.53 (0.21, 1.37) |
CMF | 6 | 22 | |||
NSABC Israel Br0283 | AVbCMF‡ | 4 | 55 | 0.91 (0.53, 1.57) | 0.88 (0.47, 1.63) |
CMF | 6 | 50 | |||
Austrian BCSG 3 | CMFVA | 6 | 121 | 1.07 (0.73, 1.55) | 0.93 (0.64, 1.35) |
CMF | 8 | 124 | |||
Combined Studies | Doxorubicin Hydrochloride-Containing Regimen | 2157 | 0.91 (0.82, 1.01) | 0.91 (0.81, 1.03) | |
CMF | 1353 | ||||
Figure 1. Meta-analysis of Disease-Free Survival
Figure 2. Meta-analysis of Overall Survival
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.