Highlights
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DEXTROSE INJECTION (25%) safely and effectively. See full prescribing information for DEXTROSE INJECTION (25%). Initial U.S. Approval: 1940 RECENT MAJOR CHANGESINDICATIONS AND USAGEDextrose Injection (25%) is indicated for the treatment of acute symptomatic episodes of hypoglycemia in pediatric patients younger than 2 years old. (1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSInjection:
CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions are hyperglycemia, hypersensitivity reactions, hyponatremia, infection (both systemic and at the injection site), vein thrombosis, phlebitis, and electrolyte imbalance. (6) To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. Revised: 7/2025 |
Indications and Usage
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
- •
- Parenteral drug products should be inspected visually for particulate matter or discoloration prior to administration. Do not use Dextrose Injection (25%) if the solution is cloudy or the seal has been broken.
- •
- Obtain blood glucose level prior to administering Dextrose Injection (25%). However, in cases of emergency, administer Dextrose Injection (25%) promptly without awaiting blood glucose test results.
- •
- Administer Dextrose Injection (25%) via slow intravenous injection into a central vein to reduce the risk of developing hyperglycemia and to minimize venous irritation [see Warnings and Precautions (5.1, 5.3)].
- •
- Do not administer Dextrose Injection (25%) simultaneously with blood through the same infusion set because pseudoagglutination of red blood cells may occur.
- •
- Administer Dextrose Injection (25%) intravenously. Do not administer Dextrose Injection (25%) subcutaneously or intramuscularly.
- •
- Discard the unused portion.
2.2 Recommended Dosage
The recommended initial dose of Dextrose Injection (25%) is 250 mg/kg to 500 mg/kg (1 mL/kg to 2 mL/kg). If clinically indicated, additional single doses of Dextrose Injection (25%) 250 mg/kg to 500 mg/kg (1 mL/kg to 2 mL/kg) may be administered.
Select the appropriate infusion rate based on the age, weight, and clinical and metabolic conditions of the patient.
Dosage Forms and Strengths
Contraindications
4 CONTRAINDICATIONS
Dextrose Injection (25%) is contraindicated in patients with:
- •
- Intracranial or intraspinal hemorrhage because Dextrose Injection (25%) can worsen cerebral edema by causing a fluid shift across the blood-brain barrier.
- •
- Severe dehydration because of the potential to worsen the patient’s hyperosmolar state.
- •
- Known hypersensitivity to dextrose [see Warnings and Precautions (5.2)].
Warnings and Precautions
5 WARNINGS AND PRECAUTIONS
5.1 Hyperglycemia and Hyperosmolar Hyperglycemic State
Significant hyperglycemia and hyperosmolar hyperglycemic state may result from too rapid administration of Dextrose Injection (25%). Symptoms of hyperosmolar hyperglycemic state include mental confusion and loss of consciousness. To minimize these risks, slowly inject Dextrose Injection (25%) and monitor blood glucose levels before and after treatment with Dextrose Injection (25%).
Due to the increased risk of hyperglycemia in neonates and low birth weight infants, use caution when selecting the dosage and injection rate of Dextrose Injection (25%).
5.2 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, have been reported with Dextrose Injection (25%) administration [see Adverse Reactions (6)]. Stop administration of Dextrose Injection (25%) immediately if signs or symptoms of a hypersensitivity reaction develop. Initiate appropriate treatment as clinically indicated.
5.3 Phlebitis and Thrombosis
Dextrose Injection (25%) is hypertonic (has an osmolarity greater than 900 mOsm/L) and may cause phlebitis and thrombosis at the site of injection. If thrombophlebitis occurs, remove the catheter as soon as possible.
Administer Dextrose Injection (25%) via slow intravenous injection into a central vein to reduce the risk of phlebitis and thrombosis. Ensure that the needle is well within the lumen of the vein and that extravasation does not occur. If thrombosis occurs, stop administration of Dextrose Injection (25%) and initiate corrective measures. If central venous access cannot be obtained in these pediatric patients, consider using an alternative commercially available dextrose product with a lower concentration.
Do not administer Dextrose Injection (25%) subcutaneously or intramuscularly.
5.4 Electrolyte Imbalance and Fluid Overload
Electrolyte deficits, particularly serum potassium and phosphate, may occur during prolonged use of Dextrose Injection (25%).
Depending on the administered volume and the infusion rate, administration of Dextrose Injection (25%) can cause fluid overload, including pulmonary edema.
Avoid Dextrose Injection (25%) in patients at risk for fluid and/or solute overload. If use cannot be avoided in these patients, monitor fluid balance, electrolyte concentrations, and acid base balance, especially during prolonged use. Additional monitoring is recommended for patients with water and electrolyte disturbances that could be aggravated by increased glucose, insulin administration, and/or free water load.
5.5 Hyponatremia
Dextrose Injection (25%) may cause hyponatremia. Hyponatremia can lead to acute hyponatremic encephalopathy characterized by headache, nausea, seizures, lethargy, and vomiting. The risk of hospital-acquired hyponatremia is increased in younger pediatric patients, and patients treated with diuretics, and patients with cardiac or pulmonary failure or with the syndrome of inappropriate antidiuretic hormone (SIADH) (e.g., postoperative patients, patients concomitantly treated with arginine vasopressin analogs or certain antiepileptic, psychotropic, and cytotoxic drugs) [see Drug Interactions (7.1) and Use in Specific Populations (8.4)].
Avoid Dextrose Injection (25%) in patients with or at risk for hyponatremia. If use cannot be avoided, closely monitor serum sodium concentrations, chloride concentrations, fluid status, acid-base balance, and neurologic status [see Warnings and Precautions (5.4)].
5.6 Neonatal Adverse Reactions from Unapproved Maternal Use at Time of Delivery
Dextrose Injection (25%) is not approved for use in adolescents or adults. Maternal hyperglycemia secondary to the use of concentrated dextrose solutions at the time of delivery has been associated with adverse outcomes such as neonatal hypoglycemia.
Adverse Reactions
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are also described elsewhere in the labeling:
- •
- Hyperglycemia and Hyperosmolar Hyperglycemic State [see Warnings and Precautions (5.1)].
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)].
- •
- Phlebitis and Thrombosis [see Warnings and Precautions (5.3)]
- •
- Electrolyte Imbalance and Fluid Overload [see Warnings and Precautions (5.4)]
- •
- Hyponatremia [see Warnings and Precautions (5.5)]
The following adverse reactions associated with the use of Dextrose Injection were identified in clinical trials or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- •
- Administration site conditions: blister, extravasation, phlebitis, erythema, pain, vein damage, thrombosis
- •
- Immune system disorders: anaphylaxis, angioedema, bronchospasm, chills, hypotension, pruritis, pyrexia, rash
- •
- Cardiovascular disorders: cyanosis, volume overload
Drug Interactions
7 DRUG INTERACTIONS
7.1 Drugs with Effects on Glycemic Control and Electrolyte Balance
Dextrose Injection (25%) can affect glycemic control, vasopressin, and fluid and/or electrolyte balance [see Warnings andPrecautions (5.1, 5.4, 5.5)]. Monitor patients’ blood glucose concentrations, fluid balance, serum electrolyte concentrations, and acid-base balance.
Concomitant administration of Dextrose Injection (25%) with drugs associated with hyponatremia may increase the risk of developing hyponatremia. Drugs associated with hyponatremia include diuretics and those that cause SIADH (e.g., selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), arginine vasopressin analogs, certain antiepileptic, psychotropic, and cytotoxic drugs). Avoid use of Dextrose Injection (25%) in patients receiving drugs associated with hyponatremia. If use cannot be avoided, closely monitor serum sodium concentrations during concomitant use [see Warnings and Precautions (5.5)].
Use in Specific Populations
8 USE IN SPECIFIC POPULATIONS
8.4 Pediatric Use
Dextrose Injection (25%) is indicated for the treatment of acute symptomatic episodes of hypoglycemia in pediatric patients from birth up to 2 years of age.
Dextrose Injection (25%) can increase the risk of developing hypo- or hyperglycemia in neonates, especially preterm neonates with low birth weight. Excessive or rapid administration of Dextrose Injection (25%) may also result in increased serum osmolality and increase the risk of intracerebral hemorrhage in very low birth weight neonates [see Warnings and Precautions (5.1)].
Monitor plasma electrolyte concentrations closely in pediatric patients who may have impaired ability to regulate fluids and electrolytes. Pediatric patients treated with Dextrose Injection (25%) are at increased risk of developing hyponatremia and hyponatremic encephalopathy [see Warnings and Precautions (5.4, 5.5)].
Dextrose Injection (25%) is not indicated in pediatric patients 2 years of age and older.
Overdosage
10 OVERDOSAGE
A medication error resulting in a high infusion rate of Dextrose Injection (25%) can cause hyperglycemia, hyperosmolality, and adverse effects on fluid and electrolyte balance [see Warnings and Precautions (5.1, 5.4)].
Severe hyperglycemia and severe dilutional hyponatremia, and their complications, can be fatal. In the event of overdosage (overhydration or solute overload) during Dextrose Injection (25%) treatment, discontinue the infusion. Institute corrective measures such as administration of exogenous insulin, and treat adverse effects on the CNS, respiratory, and cardiovascular systems [see Warnings and Precautions (5.1, 5.4)].
Description
11 DESCRIPTION
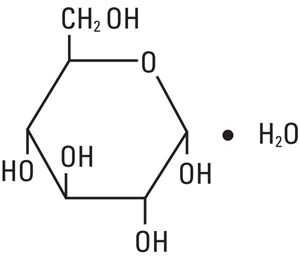
Dextrose, USP is chemically designated D-glucose monohydrate, (C6H12O6 • H2O), a hexose sugar freely soluble in water, with the following structural formula:
Water for Injection, USP is chemically designated H2O. The molecular weight of dextrose (D-glucose) monohydrate is 198.17.
Dextrose Injection, USP (25%) is a sterile, nonpyrogenic, hypertonic solution of dextrose in water for intravenous injection.
Each milliliter (mL) of fluid contains 0.25 grams of dextrose, hydrous, which delivers 3.4 kcal/gram (0.85 kcal/mL). The solution has an osmolarity of 1.39 mOsmol/mL (calculation) and the pH range is 3.2 to 6.5. May contain hydrochloric acid and sodium hydroxide for pH adjustment.
The solution contains no bacteriostatic, antimicrobial agent or added buffer (except for pH adjustment) and is supplied in a single-dose Ansyr™ Plastic Syringe.
Dextrose is derived from corn.
Clinical Pharmacology
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dextrose restores blood glucose levels and provides a source of carbohydrate calories.
Nonclinical Toxicology
How Supplied/Storage and Handling
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Dextrose Injection, USP (25%) is supplied as a clear, colorless solution in single-dose syringe as follows:
Unit of Sale | Concentration |
NDC 0409-1775-10 Bundle of 10 Ansyr™ Plastic Syringes | 25% (2.5 g/10 mL) (250 mg/mL) |
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature.]
To contact Pfizer’s Medical Information Department, please visit www.pfizermedinfo.com or call 1-800-438-1985.
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.