(medroxyprogesterone acetate)
Health Professional Information
Description
11 DESCRIPTION
Depo-subQ provera 104 contains medroxyprogesterone acetate (MPA), a derivative of progesterone, as its active ingredient. MPA is a white to off-white, odorless crystalline powder that is stable in air and that melts between 205°C and 209°C. It is freely soluble in chloroform, soluble in acetone and dioxane, sparingly soluble in alcohol and methanol, slightly soluble in ether, and insoluble in water.
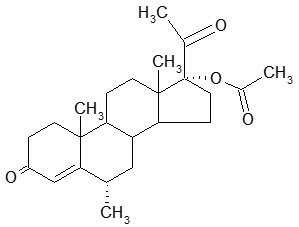
The chemical name for MPA is 17-hydroxy-6α-methylpregn-4-ene-3,20-dione 17-acetate. The structural formula is as follows:
Depo-subQ provera 104 for subcutaneous use is available in pre-filled syringes, each containing 0.65 mL (104 mg) of sterile medroxyprogesterone acetate injectable suspension.
Each 0.65 mL contains the following inactive ingredients:
Methylparaben | 1.040 mg |
Propylparaben | 0.098 mg |
Sodium Chloride | 5.200 mg |
Polyethylene Glycol | 18.688 mg |
Polysorbate 80 | 1.950 mg |
Monobasic Sodium Phosphate H2O | 0.451 mg |
Dibasic Sodium Phosphate 12H2O | 0.382 mg |
Methionine | 0.975 mg |
Povidone | 3.250 mg |
Water for Injection | qs |
When necessary, the pH is adjusted with sodium hydroxide or hydrochloric acid, or both.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.