(clindamycin)
Health Professional Information
Description
DESCRIPTION
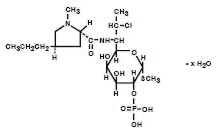
Clindamycin phosphate is a water soluble ester of the semi-synthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic lincomycin. The chemical name for clindamycin phosphate is methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α–D-galacto-octopyranoside 2-(dihydrogen phosphate). It has a molecular weight of 504.96, and the molecular formula is C18H34ClN2O8PS. The structural formula is represented below:
CLEOCIN Vaginal Cream 2%, is a semi-solid, white cream, which contains 2% clindamycin phosphate, USP, at a concentration equivalent to 20 mg clindamycin per gram. The pH of the cream is between 3.0 and 6.0. The cream also contains benzyl alcohol, cetostearyl alcohol, mixed fatty acid esters, mineral oil, polysorbate 60, propylene glycol, purified water, sorbitan monostearate, and stearic acid.
Each applicatorful of 5 grams of vaginal cream contains approximately 100 mg of clindamycin phosphate.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.