(abrocitinib)
Health Professional Information
Description
11 DESCRIPTION
CIBINQO (abrocitinib) tablets contain the free base of abrocitinib, a Janus kinase (JAK) inhibitor, for oral administration.
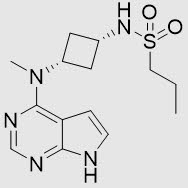
Abrocitinib is a white to pale colored powder with the following chemical name: N-((1s,3s)-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)cyclobutyl)propane-1-sulfonamide
The solubility of abrocitinib in water is 0.04 mg/mL at 25ºC.
Abrocitinib has a molecular weight of 323.42 g/mol and a molecular formula of C14H21N5O2S. The structural formula of abrocitinib is:
Each film-coated tablet contains 50 mg or 100 mg or 200 mg of abrocitinib and the following inactive ingredients: dibasic calcium phosphate anhydrous, hypromellose, iron oxide red, lactose monohydrate, Macrogol, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, titanium dioxide, and triacetin.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.