(varenicline tartrate)
Health Professional Information
Description
11 DESCRIPTION
CHANTIX tablets contain varenicline (as the tartrate salt), which is a partial nicotinic agonist selective for α4β2 nicotinic acetylcholine receptor subtypes.
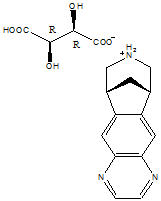
Varenicline, as the tartrate salt, is a powder which is a white to off-white to slightly yellow solid with the following chemical name: 7,8,9,10-tetrahydro-6,10-methano-6H-pyrazino[2,3- h][3]benzazepine, (2R,3R)-2,3-dihydroxybutanedioate (1:1). It is highly soluble in water. Varenicline tartrate has a molecular weight of 361.35 Daltons, and a molecular formula of C13H13N3 ∙ C4H6O6. The chemical structure is:
CHANTIX is supplied for oral administration in two strengths: a 0.5 mg capsular biconvex, white to off-white, film-coated tablet debossed with "Pfizer" on one side and "CHX 0.5" on the other side and a 1 mg capsular biconvex, light blue film-coated tablet debossed with "Pfizer" on one side and "CHX 1.0" on the other side. Each 0.5 mg CHANTIX tablet contains 0.85 mg of varenicline tartrate equivalent to 0.5 mg of varenicline free base; each 1 mg CHANTIX tablet contains 1.71 mg of varenicline tartrate equivalent to 1 mg of varenicline free base. The following inactive ingredients are included in the tablets: microcrystalline cellulose, anhydrous dibasic calcium phosphate, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, Opadry® White (for 0.5 mg), Opadry® Blue (for 1 mg), and Opadry® Clear.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.