Health Professional Information
Description
DESCRIPTION
Buprenorphine hydrochloride is a partial opioid agonist.
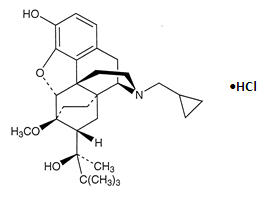
The chemical name of buprenorphine hydrochloride is 17-(cyclopropylmethyl)-α-(1,1-dimethylethyl)-4,5-epoxy 18,19-dihydro-3-hydroxy-6-methoxy-α-methyl-6,14-ethenomorphinan-7-methanol, hydrochloride [5α, 7α(S)].
Buprenorphine hydrochloride is a white powder, weakly acidic and with limited solubility in water.
Buprenorphine hydrochloride injection is a clear, sterile, injectable agonist-antagonist analgesic intended for intravenous or intramuscular administration. Each mL of buprenorphine hydrochloride injection contains 0.324 mg buprenorphine hydrochloride (equivalent to 0.3 mg buprenorphine), 50 mg anhydrous dextrose, water for injection and HCl to adjust pH to 3.5 to 5.5.
Buprenorphine hydrochloride has the molecular formula, C29H41NO4∙HCl and the molecular weight of 504.09. It has the following structural formula:
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.