(penicillin G benzathine, penicillin G procaine - BICILLIN® C-R 900/300)
Health Professional Information
Description
DESCRIPTION
Bicillin C-R 900/300 (penicillin G benzathine and penicillin G procaine injectable suspension) contains the equivalent of 900,000 units of penicillin G as the benzathine and 300,000 units of penicillin G as the procaine salts. It is available for deep intramuscular injection.
Penicillin G benzathine is prepared by the reaction of dibenzylethylene diamine with two molecules of penicillin G. It is chemically designated as (2S ,5R,6R)-3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with N,N'-dibenzylethylenediamine (2:1), tetrahydrate. It occurs as a white, crystalline powder and is very slightly soluble in water and sparingly soluble in alcohol. Its chemical structure is as follows:
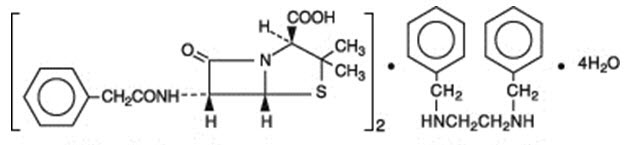 | |||
Molecular Formula | Molecular Wt. | ||
Penicillin G procaine, (2S ,5R,6R)-3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with 2-(diethylamino)ethyl p-aminobenzoate (1:1) monohydrate, is an equimolar salt of procaine and penicillin G. It occurs as white crystals or a white, microcrystalline powder and is slightly soluble in water. Its chemical structure is as follows:
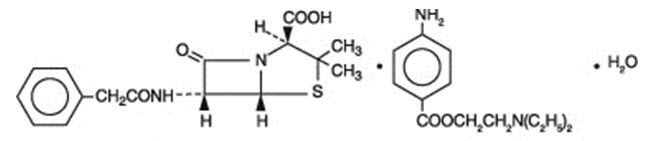 | |||
Molecular Formula | Molecular Wt. | ||
Each 2 mL syringe contains the equivalent of 1,200,000 units of penicillin G as follows: penicillin G benzathine equivalent to 900,000 units of penicillin G and penicillin G procaine equivalent to 300,000 units of penicillin G in a stabilized aqueous suspension with sodium citrate buffer; and as w/v, approximately 0.5% lecithin, 0.55% carboxymethylcellulose, 0.55% povidone, 0.1% methylparaben, and 0.01% propylparaben.
Bicillin C-R 900/300 injectable suspension is viscous and opaque. Read CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, and DOSAGE AND ADMINISTRATION sections prior to use.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.