(aztreonam for injection, USP)
Health Professional Information
Description
DESCRIPTION
Aztreonam for Injection, USP contains the active ingredient aztreonam, a monobactam. It was originally isolated from Chromobacterium violaceum. It is a synthetic bactericidal antibiotic.
The monobactams, having a unique monocyclic beta-lactam nucleus, are structurally different from other beta-lactam antibiotics (eg, penicillins, cephalosporins, cephamycins). The sulfonic acid substituent in the 1-position of the ring activates the beta-lactam moiety; an aminothiazolyl oxime side chain in the 3-position and a methyl group in the 4-position confer the specific antibacterial spectrum and beta-lactamase stability.
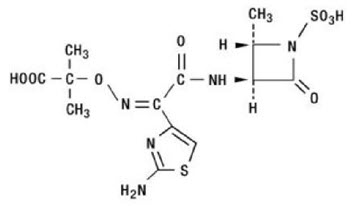
Aztreonam is designated chemically as (Z)-2-[[[(2-amino-4-thiazolyl)[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl]methylene]amino]oxy]-2-methylpropionic acid.
Structural formula:
Aztreonam for Injection is a sterile, nonpyrogenic, sodium-free, lyophilized, off-white to slightly yellowish solid, containing approximately 780 mg arginine per gram of aztreonam. Following constitution, the product is for intramuscular or intravenous use. Aqueous solutions of the product have a pH in the range of 4.5 to 7.5.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Additional Resources
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine or vaccine.
Speak with a Pfizer Medical Information Professional regarding your Pfizer medicine or vaccine inquiry.
Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for a Pfizer medicine or a vaccine.
The submission will be reviewed during our standard business hours.
To report an adverse event related to a Pfizer product and you are not part of a clinical trial* for this medication, click the link below to submit your information:
Pfizer Safety Reporting Site
*If you are involved in a clinical trial for either product, adverse events should be reported to your coordinating study site.
If you cannot use the above website to report an adverse event related to a Pfizer medication, please call (800) 438-1985.
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or by calling (800) 332-1088.